Preparing the lifETIME CDT proposal – Industry Day
After being shortlisted to prepare a full proposal, we organised an “Industry Day” on June 2018 to stimulate further collaboration around the proposal and to explore issues of importance to the bid. The feedback from the day was critical to guide many aspects of our final CDT proposal.
We hosted this event at the University of Glasgow, with sixty-two attendees, thirty-two of which were from industry and NGOs. The day was organised in two parts, a morning with presentations relating to the CDT proposal and the afternoon devoted to an Open Space discussion session. The meeting was held under the Chatham House Rule and a report was shared with all participants with outputs from discussions throughout the day.
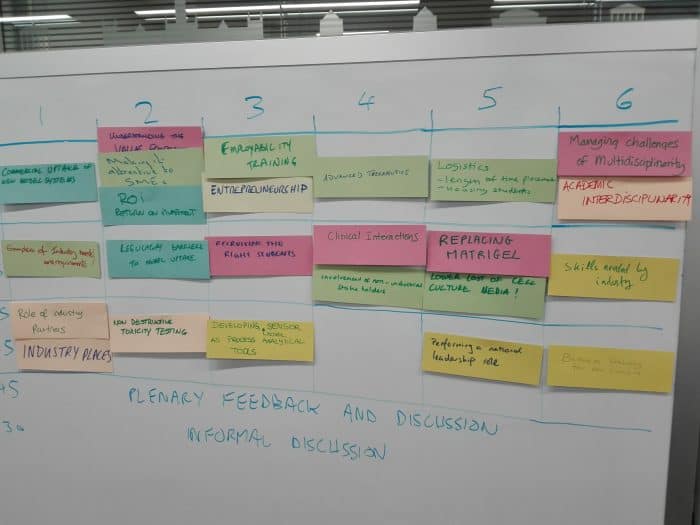
Attendees identified and discussed 23 topics of importance for the CDT:
- Commercial uptake of new model systems
- Understanding the Value proposition / Return on investment / Making it attractive to SMEs
- Employability training / Entrepreneurship
- Advanced therapeutics
- Logistics challenges (length of time placement, housing and moving students, different places)
- Managing challenges of multidisciplinarity / Academic interdisciplinarity
- Linking needs and pains to expertise and resources
- Application to cardiovascular devices
- Examples of industry needs and requirements
- Regulatory barriers to model uptake
- Recruiting the right students
- Clinical interactions / Involvement of non-industrial stakeholders
- Replacing Matrigel / Lower cost of cell culture media
- Skills needed by industry
- Key barriers between industry and academia (engagement)
- Communication within CDT
- Role of industry partners / Industry places
- Non-destructive toxicity testing
- Developing novel sensor as process analytical tools (Bio-sensing)
- Performing a national leadership role
- Business training for the cohort
- Intellectual property
- Definition of projects
The day was much more intense than we had anticipated, and it took us several days of work afterwards to process all the inputs received and the new ideas brewing in our minds. It also came up to be an excellent networking day for the attendees all in the biomedical field, networking with us but also in between the institutions and companies in the event themselves. We believe the CDT could work as a strong partnering tool for all its stakeholders and we will use the CDT events to foster this as well.
Andres Alba-Perez