Of mice and men: human ‘joint-on-a-chip’ looks to solve the puzzle of joint diseases
By LifETIME CDT Student: Abigail Wright (University of Birmingham)
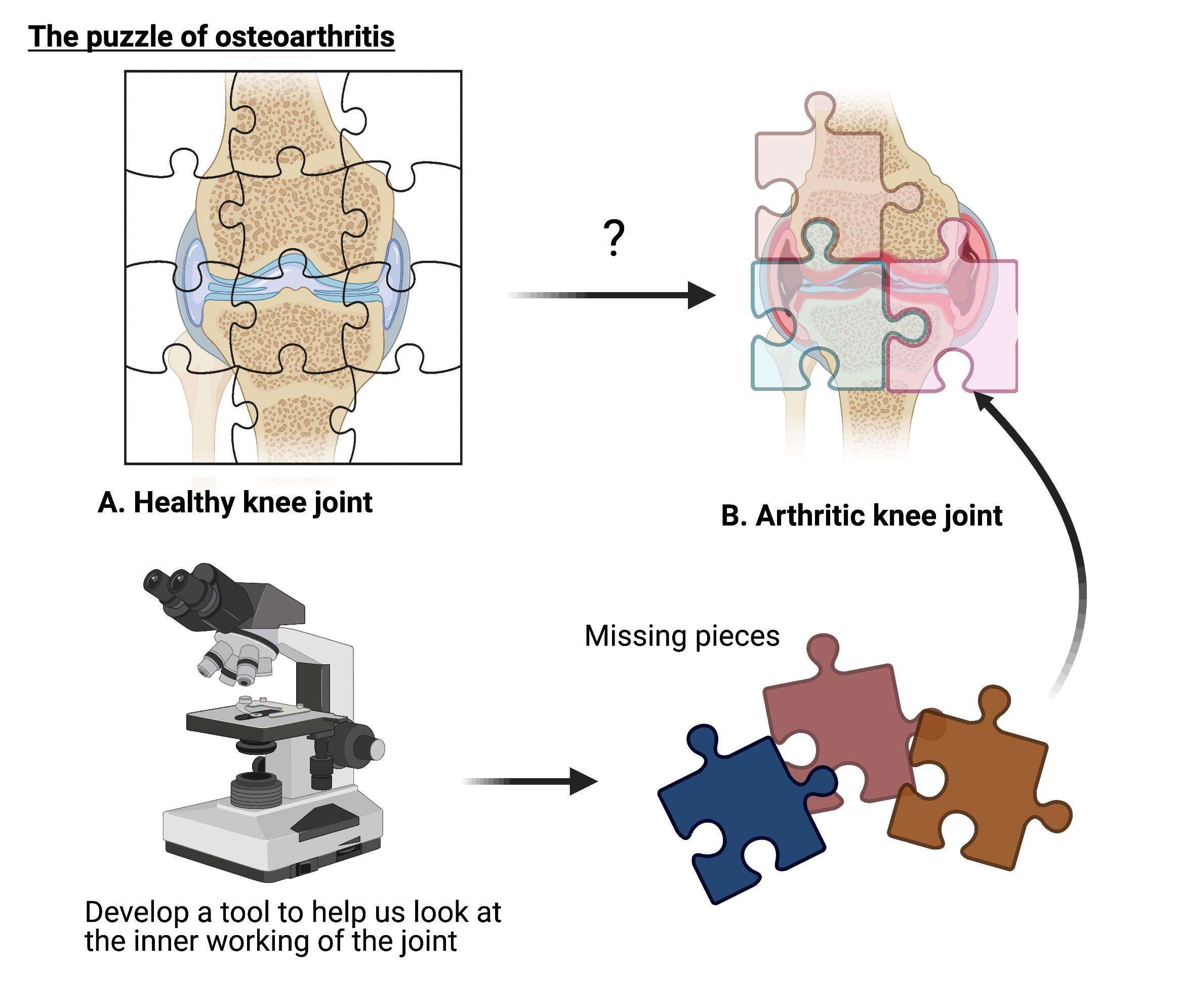
Osteoarthritis (OA) refers to a group of debilitating diseases that prevent joints from working correctly. This is the main cause of adult disability. Despite this, we are unable to treat the disease and most patients find pain management ineffective. This is due to our limited understanding how cells change during OA. Will a ‘joint-on-a-chip’ be the key to helping us solve this puzzle?
Osteoarthritis (OA) refers to a group of debilitating diseases effecting the joint, commonly manifesting in chronic pain and reduced mobility. OA effects around 250 million people worldwide and is the most common contributor to global adult disability. OA is a progressive disease, increasing in severity overtime. Outlook is poor as it is anticipated that its impact will further increase, as populations continue to age.
A key reason for this is due to our limited understanding of the disease, such as how the cells in the joint change during the disease. This makes it difficult to effectively diagnose, treat, and monitor patient with OA. The World Health Organisation has highlighted the importance of addressing these issues through fundamental research, to prevent the anticipated increasing burden on individuals and healthcare systems.
Disease modelling is a crucial tool in disease research. By mimicking diseases in the lab, it is possible to understand how different changes in cells and tissues cause diseases such as OA. However, there is a reliance on animal models to predict what will happen in humans. Evidence now highlights the inability of animal models to do this. Stark differences in non-human biology mean that data generated from animal models cannot be translated into humans. This is a key explanation as to why treatments, developed from data generated in animal models, are not effective in clinical trials. The main reason for the use of animal models has been due to a lack of alternative ways to model human diseases. Now the limitations of these models are understood, novel ‘organ-on-a-chip’ (OoAC) platforms have been developed in response.
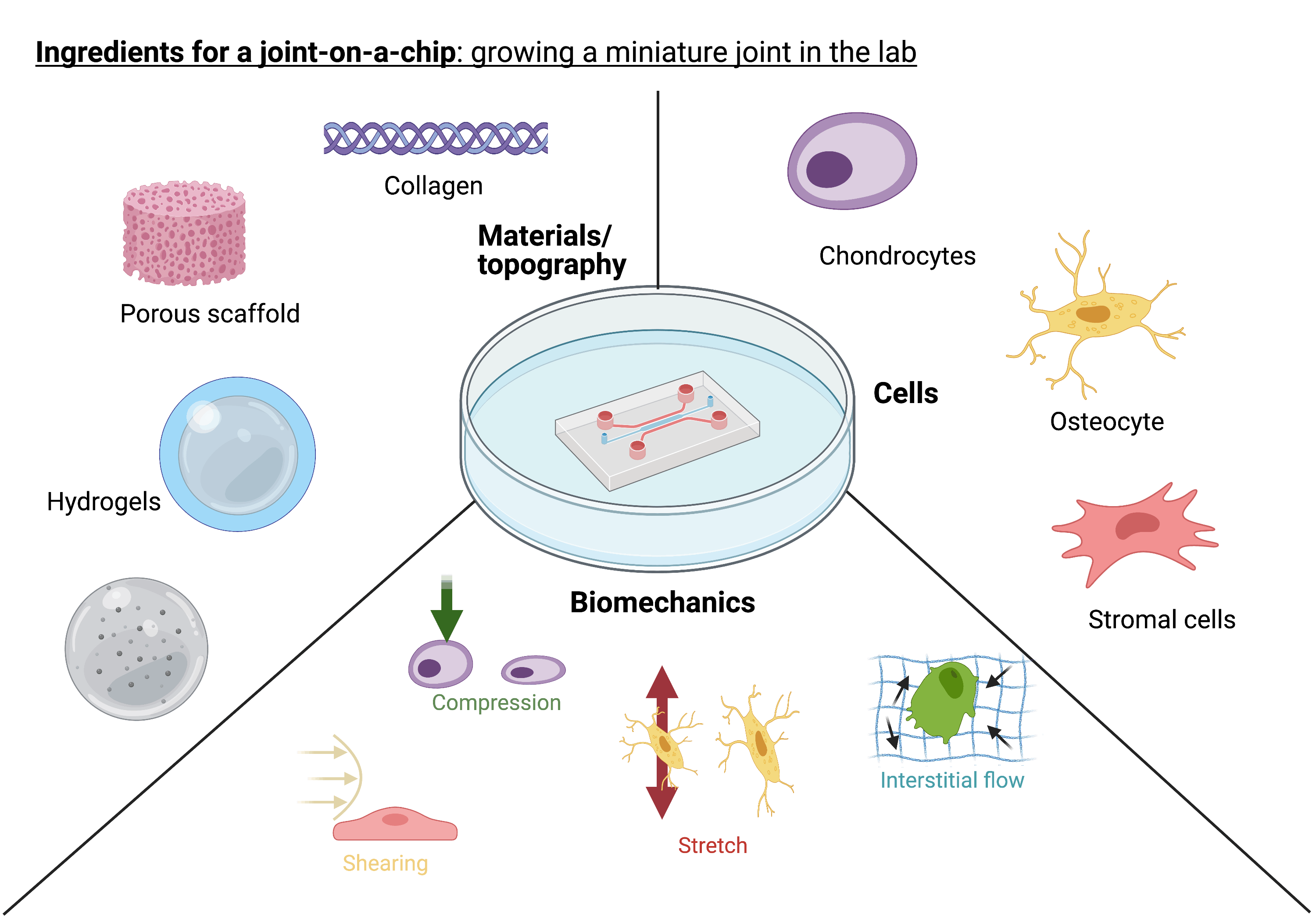
OoAC systems are small (around 250mm x 250mm) plastic ‘chips’. These systems are designed to allow us to peer inside the complex human body and understand its inner workings, to an extent not previously attainable. It is anticipated the use of ‘on-a-chip’ models will revolutionise research. Their development has required the effort of researchers across disciplines, and the concept was approached with the aim to engineer biology. In this way, we can ensure that we are replicating what we see happening in the body within the lab. Each OoAC is designed to mimic a specific tissue environment. In our bodies, cells exist within a multifaceted environment, comprising different chemicals, proteins, cell populations, and mechanics. We now understand cells are able to sense and respond to these cues and are essential to facilitating functionality of tissues. Within OoAC platforms, these complex environments can be recapitulated through specific designing and bioengineering of a ‘chip’. In this way, miniature organs can be grown in the lab and used in disease research and drug screening, instead of non-human animals. OoAC models have allowed researchers to harness tissues that work in a similar way to what happens within the body. In this way, more accurate predictions of human cellular responses and behaviour can be made, compared to animal models. Many different research groups have their own unique chip designs, depending on the tissue or organ they are recreating. The aim of my project is to develop a ‘joint-on-a-chip’ – a functioning, microscale human joint, which is yet to be achieved.
The joint is a multi-tissue system that works as a synergistic unit, allowing us to move two adjacent bones. My model must be able to mimic these different tissue environments. There are many ingredients that are required to build an environment that mimics what is seen in the body. For example, bone cells require a strong, stiff material to grow on. Cartilage cells prefer a softer more malleable material environment. These differences in material properties can have a big impact on how cells behave. Cells of joint tissues, such as bone and cartilage, are known to be able to sense and respond to mechanical stimuli. Owing to its main function of facilitating movement, a model of the joint must mimic its mechanical functionality. By establishing a ‘joint-on-a-chip’ that emulates the cell environment in health and disease, we can determine how cells change in response to different stimuli and identify how this can contribute to OA. We can identify new targets for drug development and screen them using a ‘OA-on-a-chip’ model, to predict what may happen within the human body. It is anticipated that these ‘chip’ systems will be revolutionise fundamental research and drug discovery processes. Leading the way to helping us solve the puzzle of OA and other currently untreatable diseases.