Your vision, our mission: An eye model for therapeutic development
By LifETIME CDT Student: Santino Chander (Aston University)
The eye is our window to the world. It is a vital organ that allows us to see our surroundings and loved ones. However, like all biological tissue, it can become compromised – such as through infection or a disorder such as dry eye disease. Often these compromises lead to chronic pain and affect one’s vision causing blurriness, or in severe cases, blindness. Losing the sense of vision or having chronic eye pain can be impactful on one’s quality of life and can be incredibly isolating.
One way to combat these issues is through rectifying therapeutics, but those require extensive testing on representative models – a model is an experimental system that mimics a biological system, tissue, or disease. Therapeutic development takes many years and often uses animal models due to the need for efficacy testing requirements and to ensure safety. Despite some success, these ocular animal models do not always accurately portray the human eye; differences in anatomy can affect how treatments respond, extending the process. There are also ethical issues and environmental concerns when using animal models as the process includes nurturing and then potentially harming the animal. So, what can we do to help? We believe that creating a more physiologically representative model of the whole anterior eye, whilst reducing animal usage, can be advantageous in ocular therapeutic development.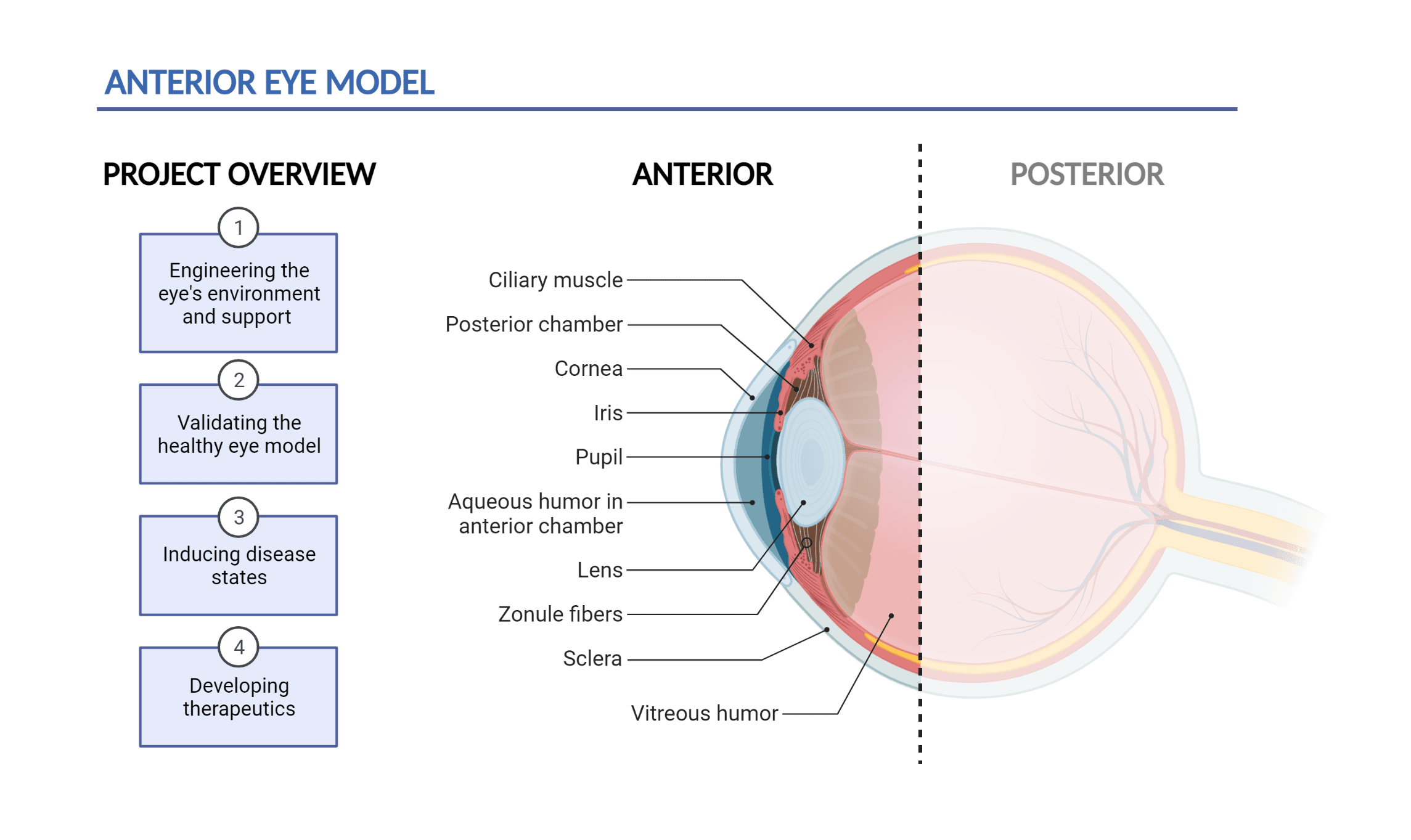
Figure 1: Project overview and anatomy of the anterior eye: The front-most region of the eye that encompasses the cornea, anterior chamber, and the lens.
In this project, we aim to create a 3D anterior eye model to aid in the development of new therapeutics and lens technologies such as eye drops, contact lenses and intraocular lenses. The anterior eye encompasses the cornea, the window of the eye, and the crystalline lens, which allows us to focus at different distances when we are younger (Figure 1). Previously these structures have been maintained in isolation but not together. Porcine (pig) eyes are used due to having similar anatomy to human eyes and are obtained as waste products from meat consumption. In the first phase of the project, we will be producing an environment that can support and maintain the physiology of the porcine eye – building a “body” for the eye so that it remains healthy for several days. This supporting construct includes a chamber to house the eye, fluidics to supply biological fluids like tears, and mechatronics to bring about physiologically relevant processes such as blinking (Figure 2). Once supported, the eye can be characterised through a battery of biological assays to assess health and function. A multidisciplinary approach will allow the assessment of parameters throughout the experiment, such as biomarkers of inflammation, the morphology of the tissue, and ophthalmologic observations such as lens power – an eye test. These results will help guide and optimise the model – for example, if the surface of the model’s eye is too dry, we can adjust the flow rate or composition of the tear fluid. We hope to keep the eye tissue healthy for at least 10 days to thoroughly test therapeutics.
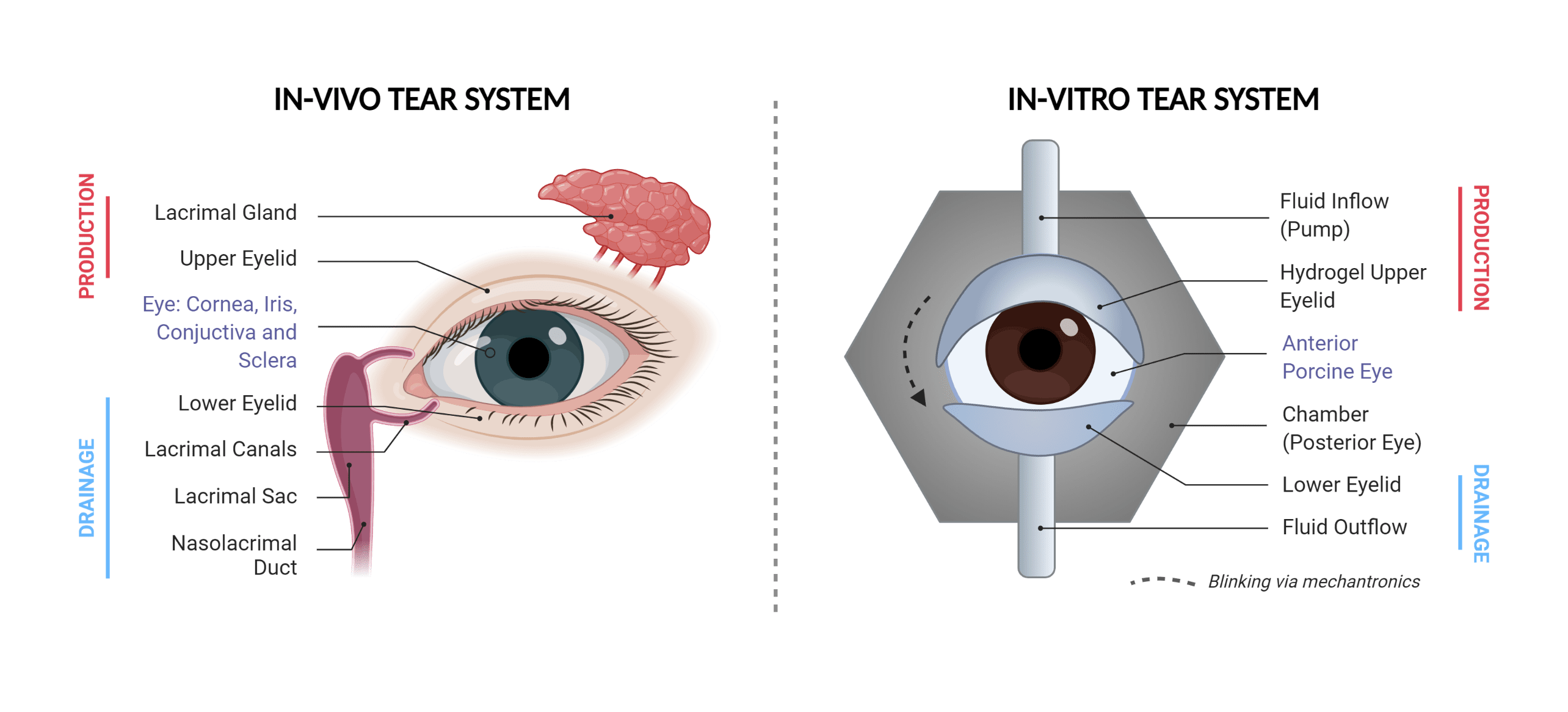
Figure 2: Schematic of the engineered in-vitro tear system: Left shows the anatomy of the body’s tear system. Right shows a schematic of the engineered tear system for our model. Components involved in tear production and drainage are labelled.
Later phases will include the induction of the disease states of the anterior eye; dry eye disease, a disorder characterised by an inadequate quantity or rapidly drying of the tear film, and presbyopia, which is the loss of ability to focus your eyes as you age due to lens rigidity. Once established, we can test therapeutics that are designed to rectify these disease states and assess drug pharmacokinetics – how the drug behaves once it has been administered. One treatment includes the use of intraocular lenses; these are synthetic lenses that can be implanted to replace a compromised crystalline lens, such as a cataract lens. An eye model could help optimise the production of these lenses, fine-tune the surgical process, and help train surgeons to administer these treatments.
With an established representative anterior eye model, we hope to improve ocular drug development by reducing inaccurate results throughout testing phases, as well as removing the need for animal testing that can be seen as unethical and environmentally impactful. Furtherly, we hope the eye model can be used as a foundation to investigate and alleviate other diseases of the eye.
Images created with BioRender.com