“Osteoporosis, I have a bone to pick with you!” Developing bone: a small molecule approach
By LifETIME CDT Student: Francesca Kokkinos (She/Her) (University of Glasgow)
Osteoarthritis, osteoporosis, and osteonecrosis are just some of the degenerative and deformative conditions which affect over 13 million people in the UK alone. All of these conditions are rooted by one small word: osteo, the Greek word for bone. Current gold-standard therapeutic strategies for treating bone defects include bone grafting whereby cell-containing bone tissue taken from the same individual is used to replace diseased or injured bone. However, bone grafting can lead to temporary changes in the donor site’s bone structure as well as lead to infection, disease and often morbidity.1,2Accompanied with the limited supply of such bone, the increasing costs and operation times, a better alternative is urgently required.
Stem cells found in the bone marrow called mesenchymal stem cells (MSCs) have been making waves in the world of regenerative and restorative medicine for their pluripotency i.e. their capability to differentiate into a number of various cell lines such as bone, tendon, muscle, fat, and cartilage. 1,3 This ability to self-renew has made them promising therapeutic tools that can be used for the treatment and correction of degenerative bone conditions through the regeneration of defected bone tissue.
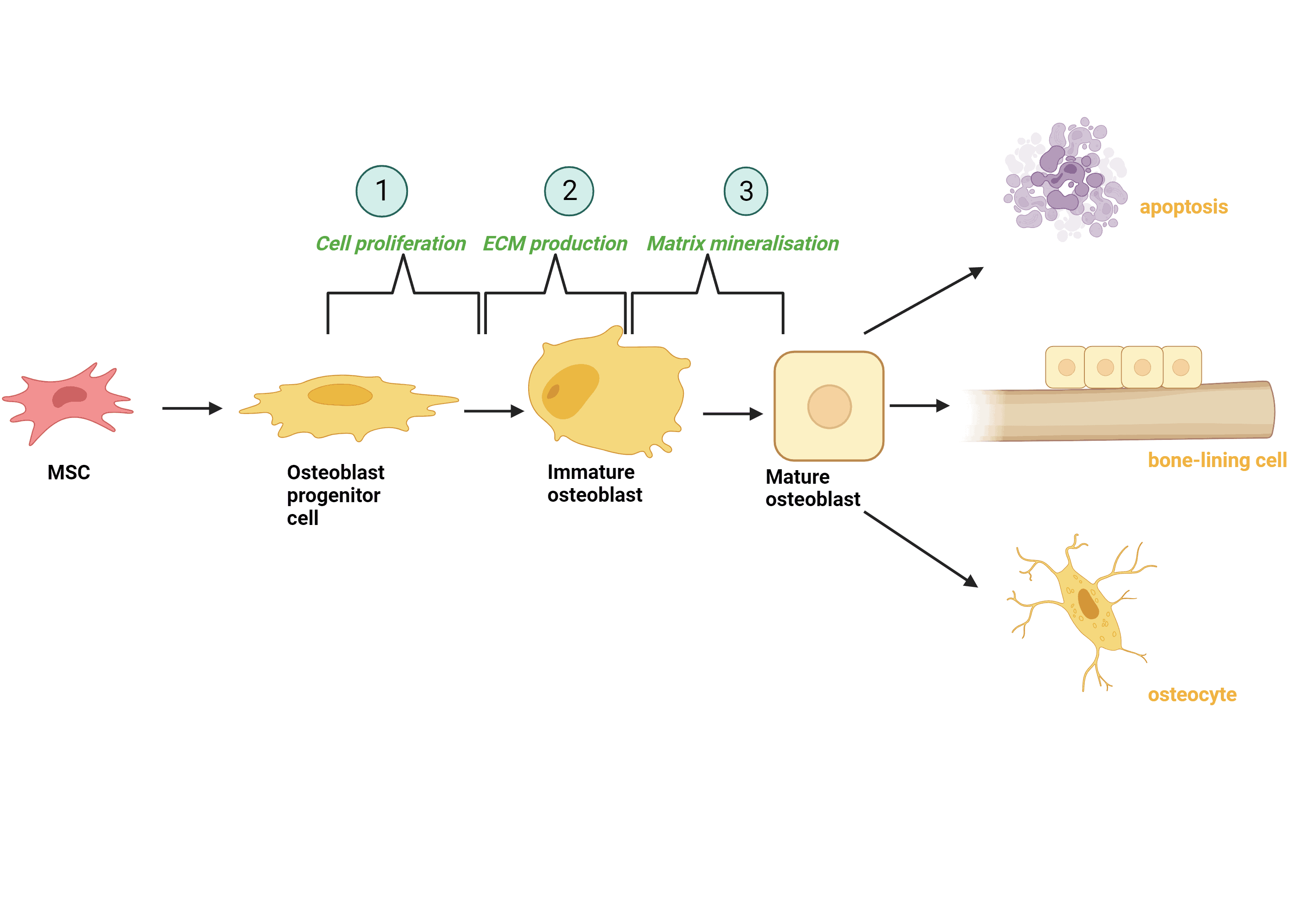
MSC differentiation of osteoblasts, cells that can both form new bone and grown and heal existing bone, consists of three main stages of development. Firstly, as osteoblast progenitor cells, they divide and multiply (proliferation) into immature osteoblasts. The next stage is where production of extracellular matrix occurs. This acts as a structural support network for the cells that is made up of many different components such as proteins and sugars thus having a vital role in a number of processes the cell undergoes, including differentiation. Mature osteoblasts take on a cuboidal shape once matrix mineralisation (osteoblast markers are produced) occurs. These mature osteoblasts can then undergo cell death (apoptosis), become bone-lining cells, or integrate themselves into the existing bone matrix as osteocytes where they can then help in bone matrix maintenance. 3
Currently, the specific osteogenic differentiation of MSCs in a laboratory setting is controlled through the addition of foreign growth factors to the media added to cells for growth. This is due to their essential role in signalling pathways important to cell differentiation and specifically osteogenesis. However, the addition of such growth factors greatly increases the likelihood of off-target differentiation that is not osteogenic. In addition, they cannot be used clinically as pharmacological doses can cause a number of side effects4.
Small molecules may provide a better alternative to the use of biological reagents, such as growth factors, for the osteogenic differentiation of MSCs. Since small molecules can also affect signalling pathways and the expression of genes in MSCs for osteogenesis, they could be a great driving force for selective differentiation. Since these are chemically synthesised and modified, their selectivity and potency can be greatly improved. A number of small molecules, known as corticosteroids, are currently in use, however, just as with growth factors, their use greatly increases the production of undesired off-target differentiation. Pharmacological doses of these molecules also have a large number of side effects once again limiting their pharmacological use. There is therefore increasing interest in the discovery of new candidate bioactive small molecules that can drive osteogenesis.4,5
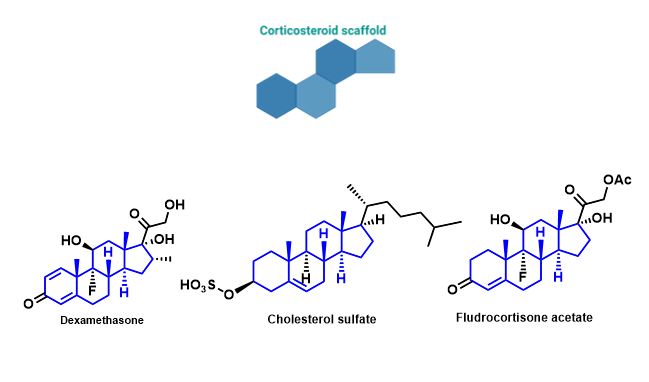
Figure 2-Molecular structures of small molecules (corticosteroids) currently in use/identified for their potential to induce osteogenesis in MSCs.
This project aims to design and synthesise new bioactive small molecules which can then be tested in a biological setting for their ability to induce osteogenic differentiation of MSCs with high specificity and potency. Such bioactive stimulators of osteogenesis in MSCs are identified through metabolomic analysis (the study of the biologically active substrates and products of metabolism which drive a wide range of essential cellular functions and biochemical process in cells that can influence their behaviour). My project aims to improve the potency and specificity of some of these identified molecules that are present endogenously and promote MSC osteogenic differentiation through organic chemical synthesis.
The synthesised molecules can then be tested biologically in MSCs for their ability to induce osteogenesis. Their potency and specificity will be assessed through the identification of certain known markers that play a role in osteogenic cell signalling and growth. The potency of the synthesised small molecules will also be compared to molecules already in use for their ability to induce osteogenesis such as the synthetic glucocorticoid dexamethasone as well as to the endogenous steroid that has not been manipulated through chemical modification.
Bibliography
(1)Tsimbouri, P. M.; Childs, P. G.; Pemberton, G. D.; Yang, J.; Jayawarna, V.; Orapiriyakul, W.; Burgess, K.; González-García, C.; Blackburn, G.; Thomas, D.; Vallejo-Giraldo, C.; Biggs, M. J. P.; Curtis, A. S. G.; Salmerón-Sánchez, M.; Reid, S.; Dalby, M. J. Stimulation of 3D Osteogenesis by Mesenchymal Stem Cells Using a Nanovibrational Bioreactor. Nat Biomed Eng 2017, 1 (9), 758–770.
(2)Mao, A. S.; Mooney, D. J. Regenerative Medicine: Current Therapies and Future Directions. Proc Natl Acad Sci U S A 2015, 112 (47), 14452–14459.
(3)Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8 (8), 886.
(4)Goodarzi, P.; Alavi- Moghadam, S.; Payab, M.; Larijani, B.; Rahim, F.; Gilany, K.; Bana, N.; Tayanloo- Beik, A.; Foroughi Heravani, N.; Hadavandkhani, M.; Arjmand, B. Metabolomics Analysis of Mesenchymal Stem Cells. Int J Mol Cell Med 2019, 8 (Suppl1), 30–40.
(5)Hodgkinson, T.; Tsimbouri, P. M.; Llopis-Hernandez, V.; Campsie, P.; Scurr, D.; Childs, P. G.; Phillips, D.; Donnelly, S.; Wells, J. A.; O’Brien, F. J.; Salmeron-Sanchez, M.; Burgess, K.; Alexander, M.; Vassalli, M.; Oreffo, R. O. C.; Reid, S.; France, D. J.; Dalby, M. J. The Use of Nanovibration to Discover Specific and Potent Bioactive Metabolites That Stimulate Osteogenic Differentiation in Mesenchymal Stem Cells. Science Advances 2021, 7 (9).