Are 3D models the future for researching colorectal cancer?
By LifETIME CDT Student: Eileen Reidy (NUI Galway)
Introduction:
Colorectal cancer is one of the leading causes of cancer related deaths. Currently patients diagnosed with advanced colorectal cancer have poor 5-year survival rates. Surgery and/or chemotherapy often do not benefit these patients. Due to the complexity and diversity of late stage colorectal cancer, the effects of different cells involved in supressing the immune system are poorly understood. The aim of my project is to develop a 3D model that can look at interactions that take place between colorectal cancer cells and other immune cells to further understand what causes suppression of the immune system and cancer progression in late-stage colorectal cancer.
Why use a 3D model instead of current models?
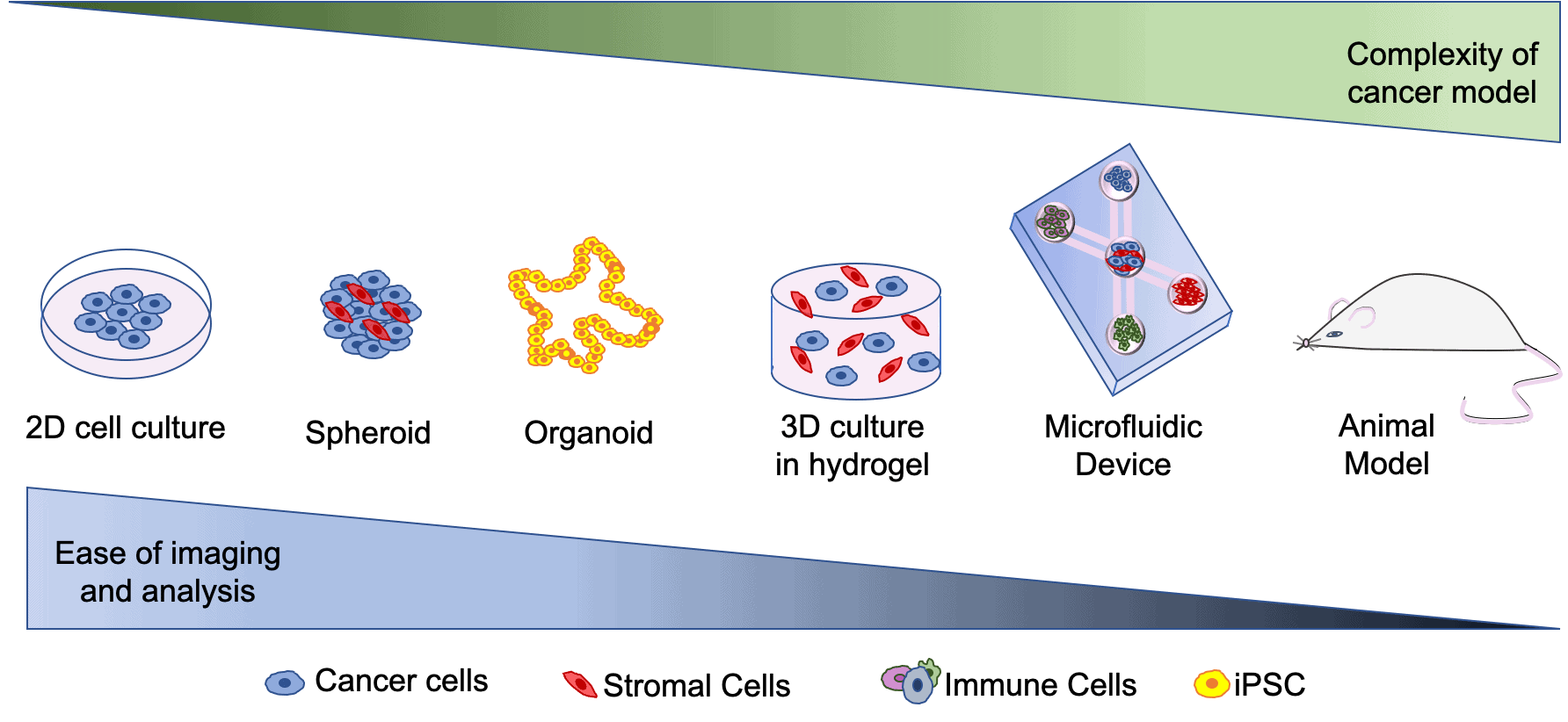
Currently there are multiple ways in which scientists study interactions that take place between cells. One of the most common methods that is used for studying cells is 2D cell culture. This involves growing cells on a flat surface in a monolayer. Although this does allow us to study some interactions that take place between cells, it does not represent the true complexity of colorectal cancer.
Often to combat this issue of complexity, scientists carry out animal studies to look at the interactions that take place at the site of the primary tumour and at different sites throughout the body as metastasis occur. Although this has many advantages over 2D cell culture, some limitations to this approach include the cost of the mouse-models and there are also problems with the sensitivity of cell imaging to investigate cell-cell interactions in the body. These limitations show the need to develop a new mode for investigating colorectal cancer.
In recent years, scientists have developed more complex models to study interactions that take place between immune cells and other cells involved in colorectal cancer. Some of these models include 3D models such as spheroids. Spheroids involve growing cells in aggregates to form a 3D clump of cells that more closely represent tumours in the body than 2D cell culture (see figure 1). In order to promote 3D growth, hydrogels (a type of polymer) can be used to allow cells keep a 3D structure. Organoids are another type of 3D model. They are developed through differentiation of induced pluripotent stem cells(iPSCs). iPSCs are cells that are capable of differentiating into all different cell types, thus they are able to be programmed to mimic colorectal cancer in-vivo. Other 3D models include ‘organ on a chip’ models that can include multiple cell types and can be used to image complex interactions that take place between different cells.
The aim of my project is to combine the two above approaches and develop a 3D ‘organ on a chip’ system, called a microfluidic device, that will incorporate multiple different immune cells, vasculature (blood supply) and primary human cells (including colorectal tumor cells). I will use this model to study the importance of different interactions that take place between immune cells and other cells involved in late stage colorectal cancer.
Figure 1. Brief overview of the 3D models of colorectal cancer.
How will the 3D model be developed?
Currently I am working on preparing spheroids that contain colorectal cancer cells and another type of cell called stromal, or support, cells. One of the prognosis factors of late stage colorectal cancer is that patients have high infiltration of stromal cells, which is why I am including this cell type. I am allowing cells to grow in different types of hydrogels including alginate and collagen, in an effort to compare differences in cell behaviours between the different hydrogels. These hydrogels will provide the cells with a scaffold in order to support their growth in 3D.
Once I have optimized this step, I will be developing a microfluidic device which I can add this spheroid to. I will then introduce other cell types including immune cells to this microfluidic device. Each different cell type will be in a different compartment of the microfluidic device (see figure 1). This device will allow me to image complex interactions that take place between cells in different compartments of the device.
Why is this important?
CMS4 is a subtype of colorectal cancer which is associated with a high infiltration of stromal cells. This subset of patients are often correlated with worse prognosis and survival rates. By developing this microfluidic device, I hope to determine the influence of stromal cell interactions on metastatic phenotype of colon cancer cells. I also hope to be able to study other complex interactions that take place between colorectal cancer cells, stromal cells and different types of immune cells. This device will increase the understanding of some of the complexity in late stage colorectal cancer. By increasing the knowledge on the complexity of late stage colorectal cancer, potential new immunotherapeutic targets for colorectal cancer may be identified.








