Can biomaterials pave the way to more advanced screening of cancer drugs?
By LifETIME CDT Student: Chanelle McGuinness (University of Glasgow)
Bone marrow is a complex organ that is home to numerous cell populations. Haematopoietic stem cells (HSCs) in the bone marrow are responsible for specialising and becoming all blood cell types – a process known as haematopoiesis. They are also the basis for HSC transplants for patients with leukaemia and blood diseases. Other cells in the bone marrow microenvironment or ‘niche’ such as endothelial cells, osteoblasts, and most importantly, the mesenchymal stem cells (MSCs), are responsible for regulating the proliferation and differentiation of HSCs. Other HSC regulatory factors in the niche include soluble signals and physical factors which vary throughout the niche, making it a very complex and dynamic environment [Figure 1]. However, this complexity is necessary to ensure tight regulation of haematopoiesis to meet physiological demands e. g. in response to stress or injury, and to maintain a consistent pool of HSCs to repopulate the blood.
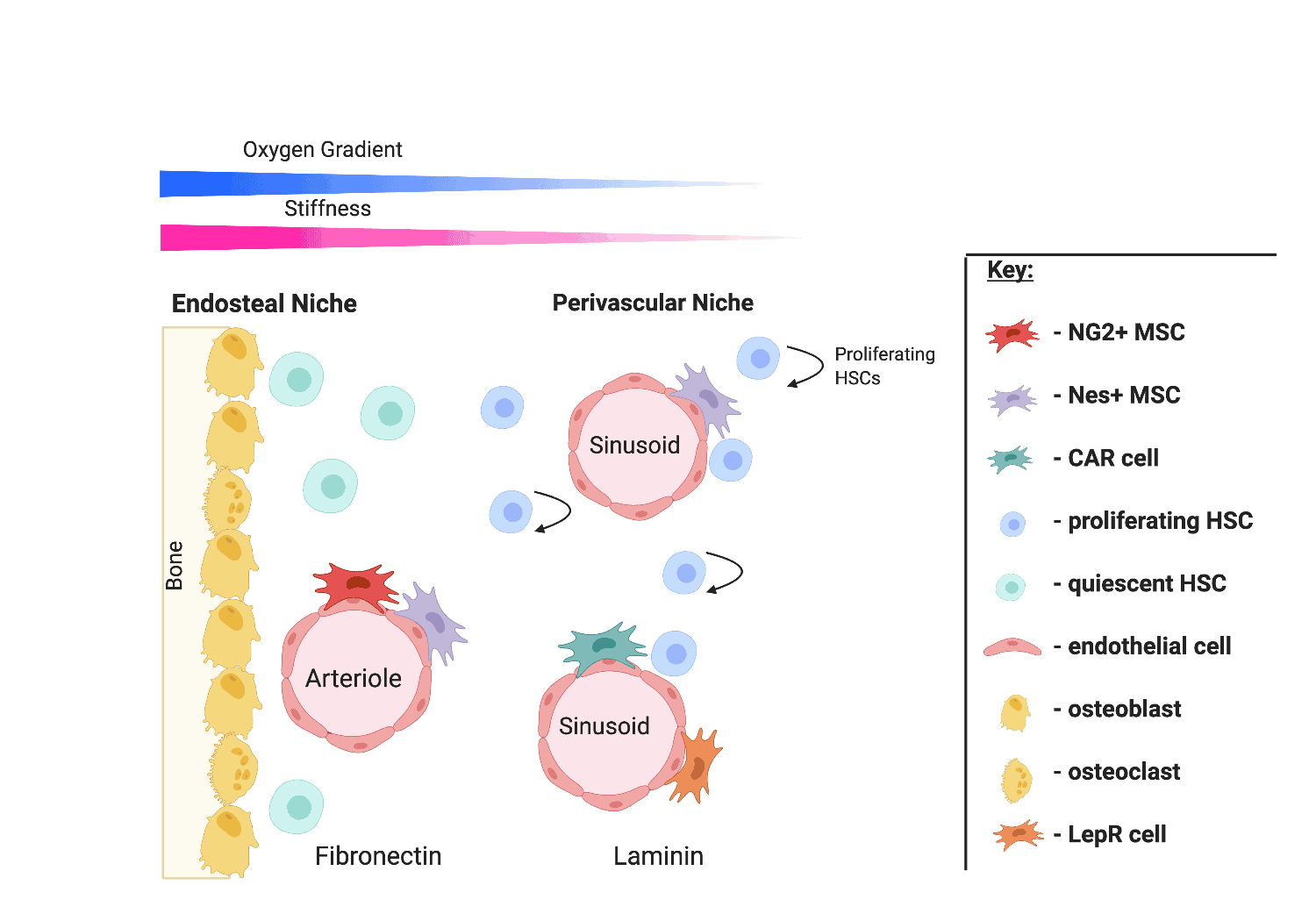
Figure 1. The bone marrow niche.
Researchers have demonstrated great interest in the bioengineering of in vitro bone marrow models out with the body (ex vivo) using biomaterials. By mimicking the bone marrow environment in a dish, this will allow scientists to investigate human haematopoiesis mechanisms, explore routes for HSC expansion for HSC transplants, explore cell-based therapies and develop drug screening platforms. The development of drug screening platforms will be the focus of my project. To date, ex vivo bone marrow model mimics can fall into three distinct categories; 2D suspension cultures that utilise supporting cell layers and growth factors, 3D cultures using hydrogels to mimic protein composition and physical aspects of the microenvironment, and highly complex micro-physiology systems (MPS) that place 3D culture models in perfusion devices to mimic blood flow, sheer stress, and provide gradients of nutrients [Figure 2].
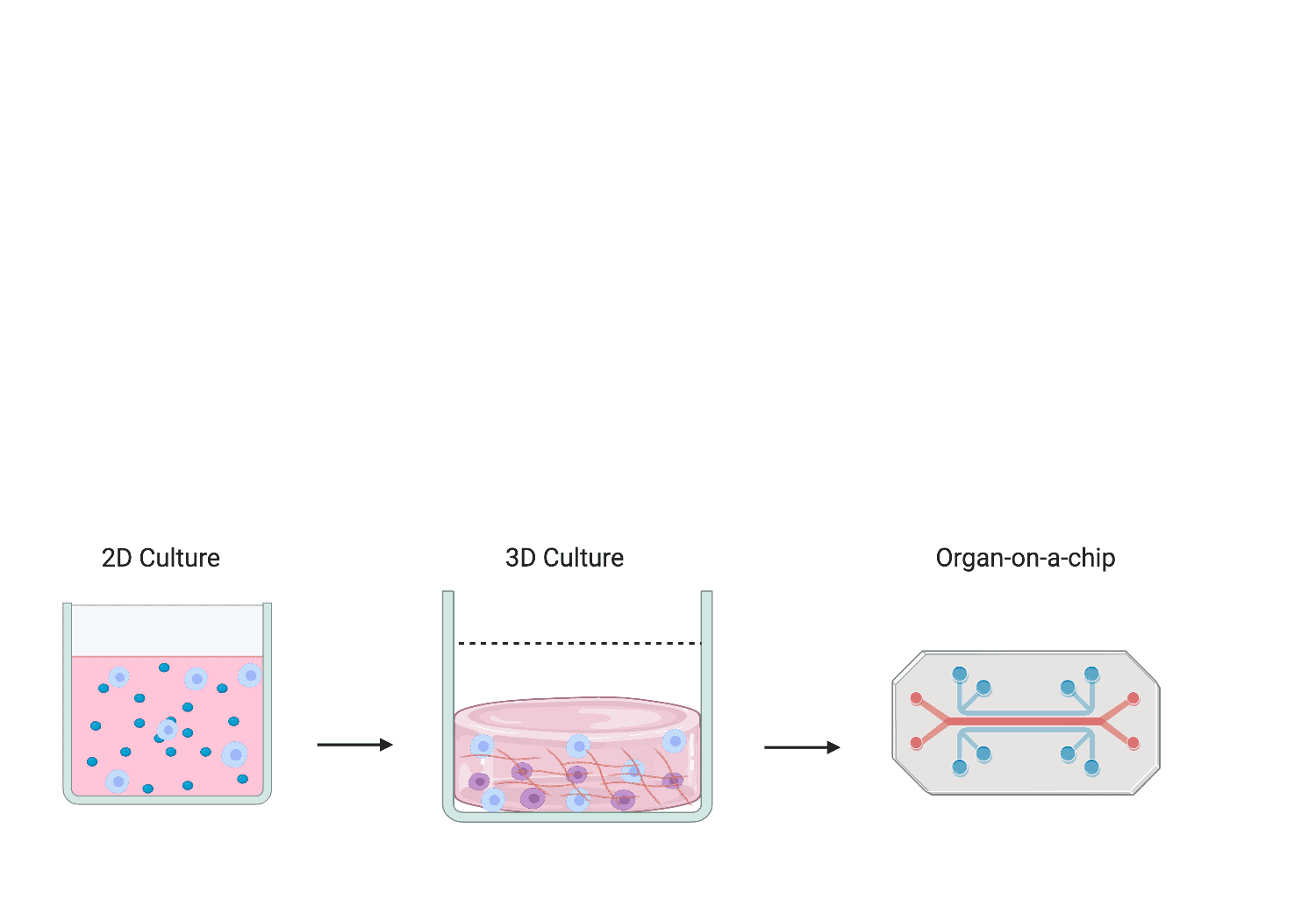
Figure 2. Schematic representation of bioengineered ex vivo bone marrow models from 2D suspension cultures to MPS.
A key part of drug discovery and development is candidate safety assessment. At pre-clinical and clinical stages, drug candidates typically go through a battery of tests to determine their potential toxicity against different organs of the body or even molecular targets such as DNA. However, considering the blood system is potentially the most well studied organ of all, the assessment of haemato-toxicity (toxicity of drugs against the blood system or bone marrow) is, in my opinion, widely neglected with negligible studies or discussions in the literature.
Cancer drugs are known to be toxic against the blood system. However, current in vitro models used in Pharma companies to identify the potential toxicity of developing cancer drugs are limited or non-existent. The few in vitro platforms used are limited as they fail to mimic the native bone marrow environment and cell viability/differentiation is problematic. Some companies choose to focus solely on animal studies for haemato-toxicity assessment. However, animal models fail to mimic human bone marrow physiology and human drug responses making them unreliable, and if drugs are proven to be toxic then the damage to animals is already done. Biomaterials offer an alternative approach for the development of more clinically relevant drug screening models by more accurately organising cells to mimic the human bone marrow environment and could allow for the prediction of potential toxicity associated with candidates prior to animal or clinical studies for adequate dosing.
My project will therefore aim to engineer an off-the-shelf bone marrow niche model that can be utilised for the sole purpose of drug screening and haemato-toxicity assessment. This is important to provide insights into the potential toxicity associated with candidate compounds to assess their continuation in development and risk: benefit. This will aid in reducing drug attrition rates in Pharma and predicting dosing concentrations and schedules in animal studies and in clinical trials. Currently, Pharma companies either rely solely on animal studies or outsource expensive and time consuming in vitro MPS models that both come with their limitations. By providing a simpler alternative model, this could be more cost and time effective and offer higher throughput of screening. In addition, my model will link with the 3R’s principle that seeks to replace, reduce and refine the use of animal models in research which is highly valued. Finally, this model could be applied in personalised medicine approaches to screen patient -specific stem cells for therapeutic responsiveness to certain drugs and appropriate treatment regimes.








