Sweet Seaweed Slumber
By LifETIME CDT Student: Juda Milvidaite (University of Glasgow)
Cells, tissues, and organs are fragile – they cannot survive outside of a living body for long. When we need to store or transport biological samples from cancer patients for further research or personalised screening of therapies, we freeze the samples down. Yet, the cycles of freezing and thawing can damage the architecture of the tissues we aim to maintain. Interestingly, encapsulation within algae-derived gel – alginate – could potentially be used as an alternative to freezing. Samples preserved within alginate are easier to handle, whether by car or via mail, as they can be shipped at ambient temperatures and there is no need for specialised vehicles or storage freezers. However, it is still unclear whether the ever adapting and shapeshifting cancer cells and tissues can be reliably stored within the alginate gel.
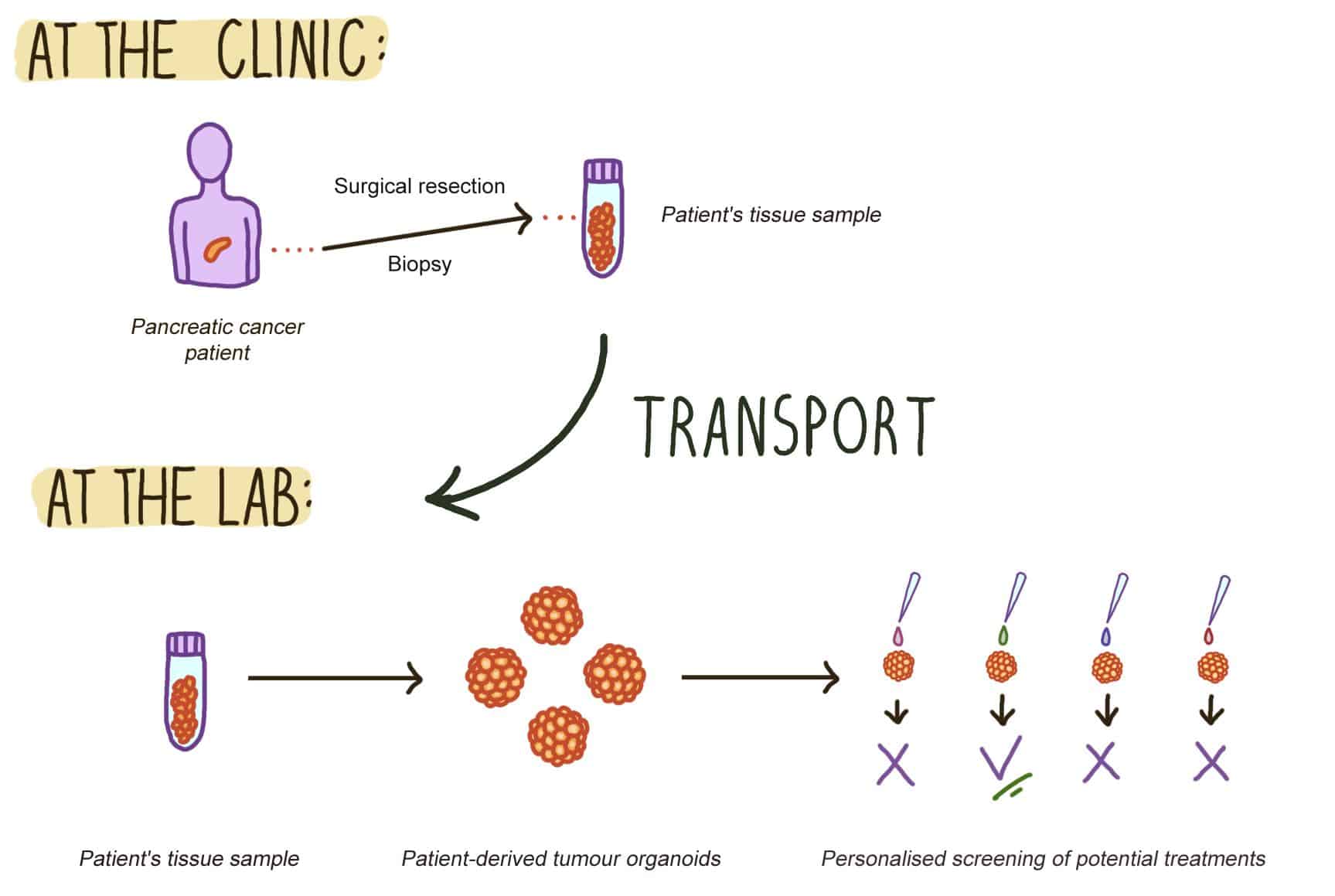
Pancreatic cancer is one of the most aggressive solid tumours. Pancreatic ductal adenocarcinoma (PDAC) describes tumours of the exocrine pancreatic tissue, that account for 90% of all pancreatic cancer cases. Unfortunately, PDAC is a highly metastatic malignancy that is often discovered at the end-stage of the disease. At this point, conventional treatments, such as surgical resection and chemotherapies mostly fail. This highlights the need for personalised drug testing platforms, that allow to quickly evaluate the most promising combination of chemotherapeutic agents or novel therapies for the individual PDAC patient. To that end, preserving primary PDAC clinical samples (e.g., biopsies and surgically resected tissues) is crucial, as these patient tissues can be used for establishing patient-specific tumour organoid models to be used in screening for treatments.
Figure 1. From the clinic to the lab: primary patient tissue samples are used for patient-derived organoid modelling. Surgically resected pancreatic tumour tissues or patient biopsy samples need to be preserved during transport from the clinic to the lab. At the lab, the primary patient tissues are used for establishing in vitro models, such as patient-derived tumour organoids, that can used for screening most efficacious cancer treatment for the individual patient.
In vivo, the tissue-specific extracellular matrix (ECM) surrounding the cells naturally contains ligands that the cells can bind for anchorage, via integrin receptors. Integrin-mediated cell-ECM interactions play a key role in cell survival, proliferation, differentiation, and migration. The plant-derived alginate matrix lacks the respective bioactive ligands required for integrin-mediated cell attachment to matrix. That is, for mammalian cells, alginate is biologically inert. It is known that the lack of anchorage to the matrix and contact inhibition from the increase of cell-to-cell interactions can result in cell cycle arrest. I.e., cells should stop growing and proliferating in alginate. However, contact inhibition is one of the anticancer mechanisms that is often lost in malignant cells. This begs the question: can PDAC samples be reliably stored in alginate? – Will they slumber? Or will the cancer cells continue growing and thriving regardless?
In this project, the biological response of both in vitro PDAC models and human clinical samples to short-term storage within alginate will be characterised. This includes foundational cell read-outs like viability and morphology, as well as quantification of gene and protein expression (e.g., measuring the levels of cell cycle genes). Metabolomic screens will also be performed to better understand PDAC sample phenotype and activity throughout 5-day storage within alginate.
Moreover, it will be essential to understand whether the physical characteristics of alginate play any role in how effective the gel encapsulation is in preserving PDAC samples. Therefore, elasticity and viscosity of alginate will be characterised, along with assessment of whether these material properties influence the biological responses by the cancer cells. It is yet unclear whether the physical properties of alginate have any effect over the success of short-term sample storage. Therefore, PDAC samples will also be stored over longer timescale (14 or 21 days) to reveal any changes to biological and material interactions in relation to time.
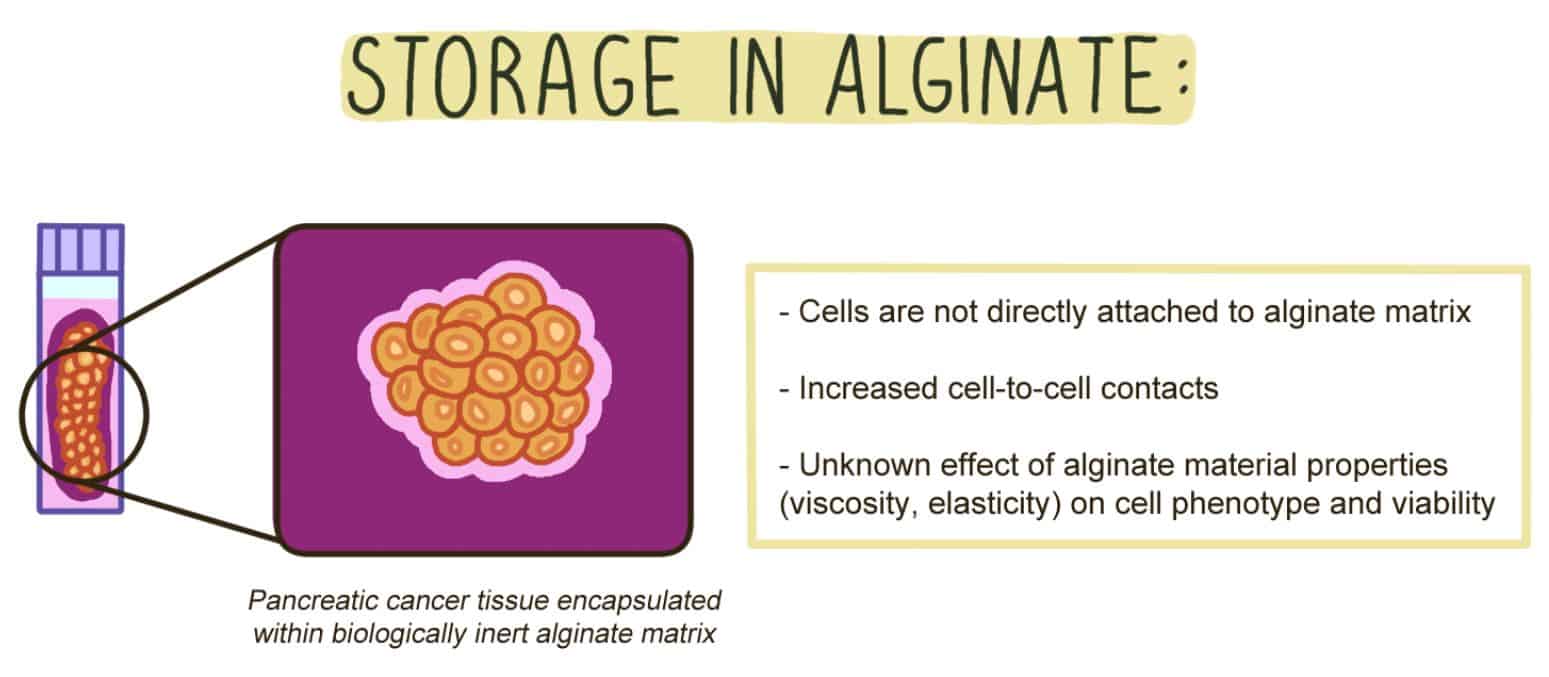
Figure 2. Cells stored in alginate do not directly adhere to the surrounding matrix.
When pancreatic cancer tissue sample is encapsulated within alginate, the cells do not form connections to the matrix. Due to the lack of cell-matrix interactions, there is an emphasis on cell-to-cell contacts. However, it is yet unclear whether physical characteristics of the alginate gel, such as viscosity and elasticity matter for the short-term storage of pancreatic cancer tissues and cells in alginate.
In short, this project will help illuminate whether solid tumour models and primary patient tissues can be successfully preserved at ambient temperatures using alginate, and the extent to which physical alginate properties matter for this purpose. Since alginate preservation is low cost and does not require specialised equipment, it would allow more researchers to make use of tumour tissues and engage in patient tissue-derived organoid modelling. Overall, reliable storage within alginate would help to optimise sample preservation from the clinic to the lab bench, from one lab to another, strengthening the networking between the clinical, academic, and industrial scientists by easing the transport and sharing of advanced in vitro models and primary patient tissues.