Decoding Mysteries of Blood-Brain Barrier
By LifETIME CDT Student: Viswanath Vittaladevaram (NUI Galway)
Like a defensive line in a football field, the blood-brain barrier protects central nervous system against pathogens and toxins and regulates the passage of many cells and substances into nervous tissue. A vast majority of disorders associated with central nervous system is linked with blood brain barrier dysfunction and few examples include Alzheimer’s, Parkinson’s and multiple sclerosis. Disorders of central nervous system affect millions of people across the world, making their day-to-day life very challenging for both patients and caretakers. At present, the treatment options are mainly focused on relieving symptoms temporarily but not able to cure the diseases and research is still in progress to unravel the mysteries of blood-brain barrier (Figure 1).
Figure 1: A Schematic representation of Blood-Brain Barrier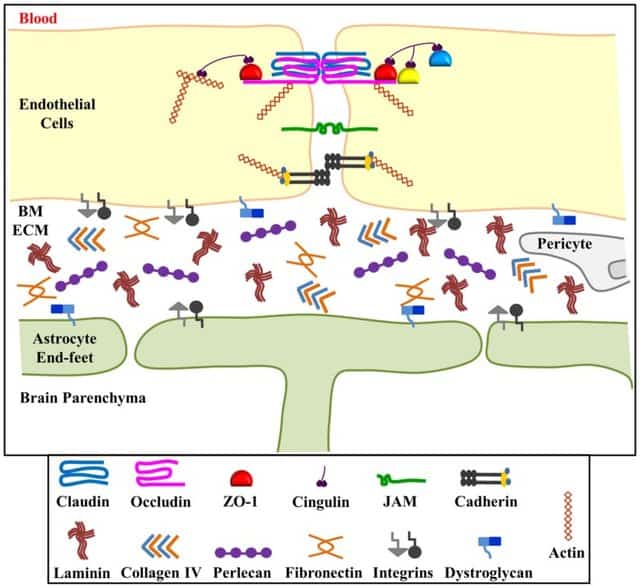
To date, a variety of blood-brain barrier models were created to find the underlying mechanism of blood-brain barrier and to screen the candidate drug molecules. But all these models were hindered by interspecies differences when translated into humans. As it is difficult to investigate the blood-brain barrier in the body we aim to develop synthetic models of it. It is essential to understand the biological, cellular and morphological properties of blood-brain barrier in terms of health and disease and to develop therapeutic drugs that can pass through the barrier.
To better understand the complexity of blood-brain barrier, researchers used computational models to investigate the pathologies such as Alzheimer’s and brain tumours etc. Computational methods were used to study the pathologies of blood-brain barrier and were aimed at drug design and delivery into brain. All these methods were used to develop more complex simulations related to patient-specific pathologies and evaluate the progression of disease. Also, to ease the process of drug delivery system into brain.
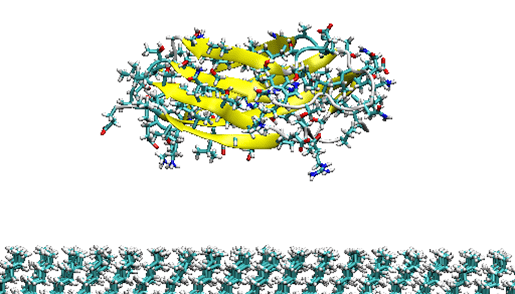
In this project, we aim to develop a synthetic blood-brain barrier model that mimics the properties of real blood-brain barrier and allows to study transport of molecules and further investigate the uptake and screening of candidate drug molecules. To optimize the formation of blood-brain barrier model, it is necessary to understand the interactions between protein adsorption onto surfaces and it is difficult to retrieve experimentally. But this can be achieved using computer modelling and simulation procedures by investigating the changes in solution conditions, protein concentration etc. that influence the structure of protein layer and optimize the of formation of blood-brain barrier model. In this study, we first investigate the adsorption of fibronectin fragments onto different surfaces using molecular dynamics simulations which is used to determine the protein layer formation on surfaces that is essential for growth (Figure 2).
Figure 2. Fibronectin fnIII-12 fragment near hydrophobic surface
Using atomic force microscopy (AFM), interactions between fibronectin fragments and different surfaces were quantified and further complemented with quartz crystal microbalance (QCM) for adsorption measurements. This will give insight into the protein molecules and surface interactions and will be used for optimization of blood-brain barrier model and further supplemented with cell growth studies. The expected outcome of this computational model will contribute to understand the formation of blood-brain barrier under normal and diseased states and eventually could lead to better treatment strategies for brain related disorders.