Engineering Biologically Active Vascular Grafts
By LifETIME CDT Student: Justine Clarke (She/Her) (University of Glasgow)
Cardiovascular diseases (CVDs) are the leading cause of death globally. They can occur in several different forms, most commonly from a build-up of fatty tissue or blood clots that restrict the blood flow to the brain, heart, or limbs. Another type of CVD occurs when the walls of the aorta are weakened and begin to bulge, causing blood flow to put strain on the vessel wall, known as an aneurysm.
For my PhD project I will be focusing on abdominal aortic aneurysms (AAAs) and investigating how we can use cells to improve current treatments. If left untreated, the consistent pressure from blood flow can cause the aorta to continuously bulge and risk rupturing (figure 1A). One of the current treatments for this involves implanting a synthetic vascular graft by keyhole surgery. The graft is compacted in a delivery tube system and fed through the blood vessels to the site of injury (figure 1B). When released, the graft expands to support the blood flow through the aneurysm, whilst allowing for pulsations to simulate the natural behaviour of the aorta (Figure 1C). Over time, however, scar tissue and blood clots can begin to form around the vascular graft which can risk blocking the blood flow.
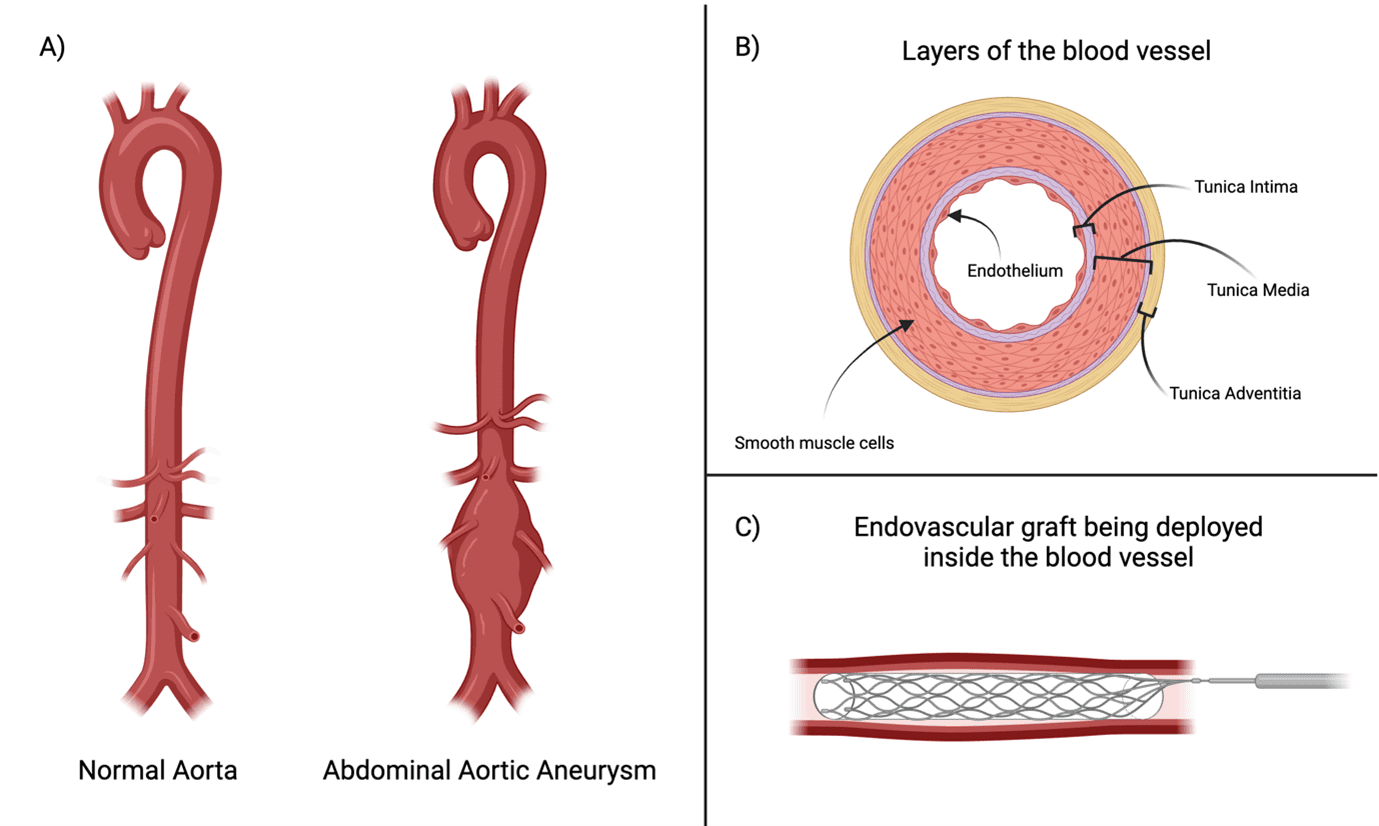
Figure 1. (A) A healthy aorta in comparison to an aorta with an abdominal aneurysm, illustrating how the aorta bulges. (B) Cross-sectional diagram of the different layers of the blood vessel. (C) An example illustration of the vascular graft within the blood vessel following implantation.
The aim of my project is to use a biological approach to encourage the vascular graft to adopt a more native structure in the blood vessel. To do this, I will be using extracellular vesicles (EVs), which are nano-sized vesicles (ranging from 30-1000nm), that are secreted by most cells and act as a signalling carrier between the parent cells and neighbouring cells within the body. EVs are known to confer the properties of their parent cell to the target cell, making them valuable in therapeutic bioapplications. EVs contain various protein and genetic information from the parent cell, which can be influenced by priming the parent cell through specific culture techniques. Mesenchymal stem cells (MSCs) are well established in regard to their regenerative capacity and immunomodulatory properties, therefore I aim to use MSC-EVs in my project, to encourage vascularisation.
For my application, I will prime my MSCs by culturing them in both normal and low oxygen levels (hypoxia), as hypoxia is reported to generate MSC-EVs with enhanced properties for promoting blood vessel formation. Subsequently, I aim to deliver my MSC-EVs by immobilisation on the vascular graft material, by a coating which opens binding sites for the integrins on the surface of EVs to adhere to (Figure 2). When the vascular grafts are implanted into the body, with the additional biological coating of EVs, the principle is that the release of EVs at the site of injury will communicate with the native endothelial cells, promoting accelerated repair of the endothelium inner blood vessel layer.
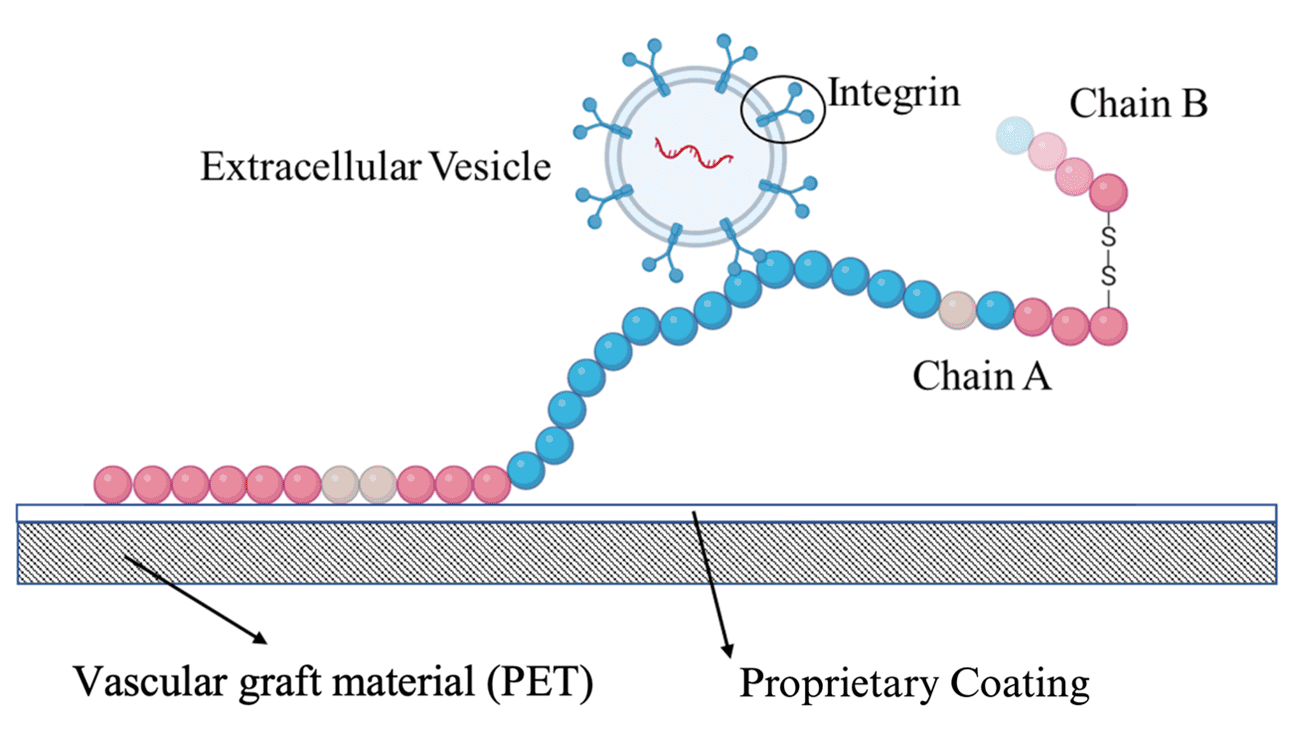
Figure 2. A schematic diagram of the protein and proprietary coating on the Polyethylene Terephthalate (PET) vascular graft material. When combined, the protein is encouraged to open out from its originally more globular form, creating nanonetworks across the graft surface that the integrins of the EVs can bind to.








