From bench to bedside: how physiological relevance underpins the future for Mycobacterium abscessus drug discovery.
By LifETIME CDT Student: Emily Baker (She/Her) (Aston University)
Think back to the last time you took a shower or did some gardening on a (albeit rare) sunny day. How did you feel? Were you worried about your wellbeing or what you may be exposed to in the process? Most people don’t think about this on a routine basis… except for if (like me) you’re a scientist who is interested in the ‘whats’ and the ‘whys’. There is a high chance that you would have encountered Mycobacterium abscessus – a type of environmental bacterium that typically lives within water sources and soil. For the healthy population, this exposure is harmless, with our functioning defence mechanisms dealing with the threat without you ever knowing. However, for the portion of the population experiencing defective lung function (including those with cystic fibrosis), this exposure can cause severe, and oftentimes fatal, lung infection. The environmental nature of this infection unfortunately means that the threat is everywhere for these individuals, making M. abscessus incredibly difficult to deal with as a patient with cystic fibrosis.
Once a patient becomes infected with M. abscessus, they will be prescribed a multi-drug antibiotic treatment regimen that typically lasts over one year. Despite the complexity and intensity of this therapy, treatment success remains incredibly low, with curative therapy only being possible for infections limited to one area of the lung – whereby surgical resection can be combined with antibiotic treatment. For this reason, there is a great need to discover novel antibiotics that can successfully treat and eradicate this bacterium with minimal treatment burden. Unfortunately, though, the antibiotic discovery sector faces multiple challenges that currently detriment the success of antibiotic discovery efforts. One such flaw includes the physiological relevance of the currently accepted laboratory practices, which is the focus of my PhD project.
Physiological relevance is a term used to describe how closely a laboratory method mimics the environment encountered within the body (or the physiological environment – hence the name). When discovering and developing new antibiotics, scientists will typically conduct their experiments in plastic plates, whereby bacteria can be combined with different antibiotic candidates and monitored over time (Figure 1). Although this provides a rapid (and relatively simple) indication of how a bacteria may respond to a novel compound, the precision of these methods is greatly flawed by their physiological inaccuracies. The complex interactions and behaviours adopted by bacteria in the body are responsible for their resistance to antibiotic treatment, which isn’t represented in these plastic laboratory plates. As such, we face problems when we try and implement a successful antibiotic candidate from the laboratory to the clinic; what may look like a great inhibitory concentration in the lab becomes far too high for safe administration when implementing the compound into the human body. One key factor implicated in this is the presence of in vivo biofilms, which is a focus of my project.
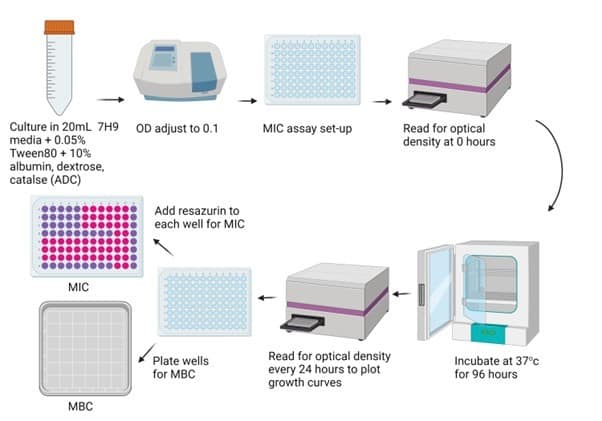
Figure 1: Workflow of conventional antibiotic screening. Resazurin is a viability indicator, where pink indicates bacterial growth. MBC: minimum bactericidal concentration; MIC: minimum inhibitory concentration.
How would you typically protect yourself from the cold in the winter? Most of us would layer up, or put a coat on, right? Think of biofilms as the coat of the bacterial world, however instead of protecting themselves from the cold, they are shielding themselves from antibiotics and the immune system. Standard bacterial cultures do not consider this biofilm phenotype, therefore greatly under-representing the resistance encountered in vivo. Although there are assays that do consider biofilms, their use of standard laboratory media mean that they fail to represent the architectural complexity of those encountered in the body. Figure 2 provides a schematic of the molecular complexity of in vivo M. abscessus biofilms, grown using media optimised to mimic the cystic fibrosis sputum.
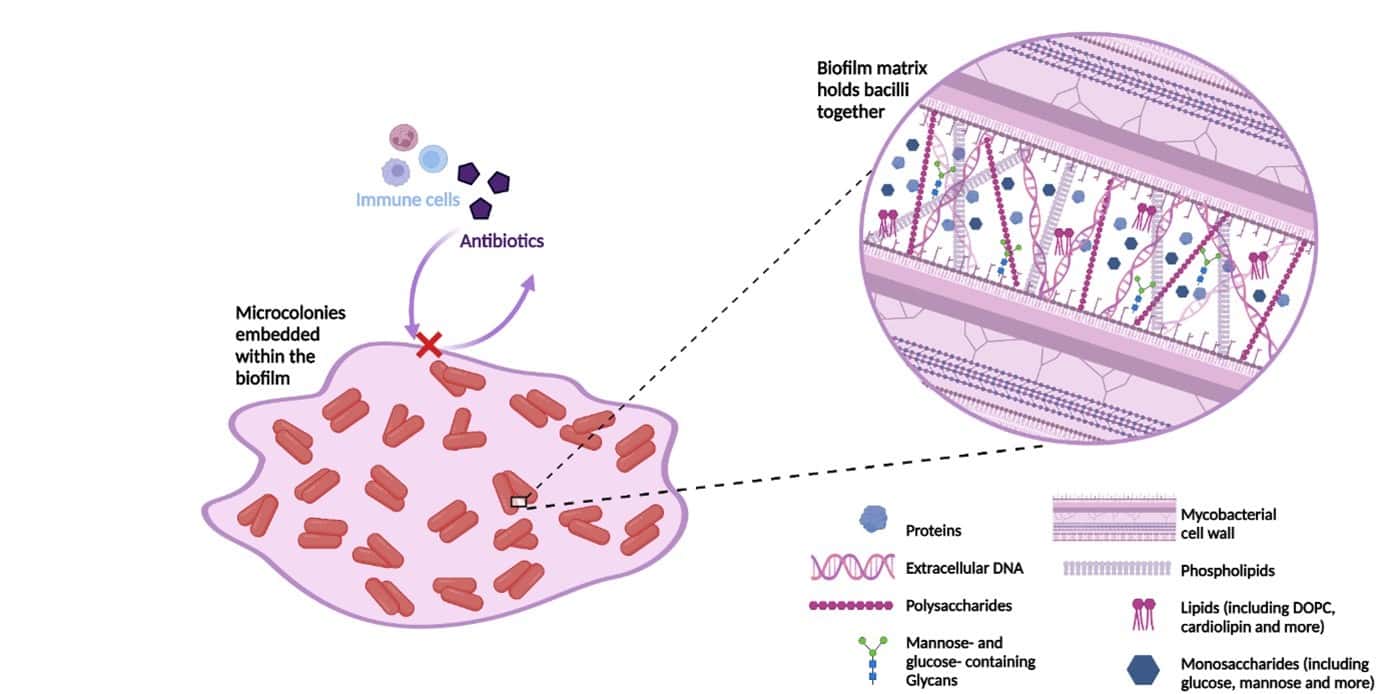
Figure 2: The molecular composition of the extracellular matrix of SCFM-grown M. abscessus biofilms. Quantities of each molecular component are not to scale but aim to demonstrate their presence within the matrix. Summarised based on the work of Belardinelli et al., 2021. Figure created on Biorender.com, accessed 16th July 2023. DOPC, dioleoyl phosphatidylcholine; SCFM, synthetic cystic fibrosis sputum media.
In my project, I hope to combine engineering approaches with conventional drug discovery to test novel antimicrobial candidates in a more physiologically relevant environment. The first year of my project has identified multiple antibiotic ‘hits’ using conventional drug screening, which we aim to translate to such system to greater understand their clinical potential.
References:
Belardinelli, J.M. et al. (2021) ‘Unique Features of Mycobacterium abscessus Biofilms Formed in Synthetic Cystic Fibrosis Medium’, Frontiers in Microbiology, 12, p. 743126. Available at: https://doi.org/10.3389/fmicb.2021.743126.