HOW TO BUILD A BRAIN: Looking to the developing brain when developing brain models
By LifETIME CDT Student: Martha Gallagher (She/Her) (Aston University)
Neurodegenerative diseases – varying in action but all resulting in the degeneration of parts of the nervous system – pose one of the greatest threats to our healthspan today. You can probably name at least one person in your life who is living with Parkinson’s, ALS, MS, Alzheimer’s, Huntington’s, other dementias etc…, and yet there is no curative treatment for any.
The therapy development pipeline is dire (mostly unsuccessful, expensive and averaging 12-15 years) but it is not for lack of trying. Many therapies, mainly drugs, have been exciting in preclinical trials only to fail in the clinic. One of the reasons for these failures can be traced back to the preclinical stages though, and the choice of disease model: the gold standard for many years has been mouse models. Other than the obvious ethical issues of using animals in research, it has also become clear that what is true in mice does not necessarily translate to humans when looking at neurodegenerative diseases.
Simply put, the gold standard is losing its shine and we need a new one quickly: current estimates suggest that by 2050, 153 million of us will have dementia.
This is the rationale for my project – we need an alternative for animal models, cells in plastic flasks don’t experience anything close to the complex 3D environment of the brain, and now we have induced pluripotent stem cells, theoretically you can build a patient specific model, complete with a 3D microenvironment and neural cells that contain the same genetic pattern of disease as the patient.
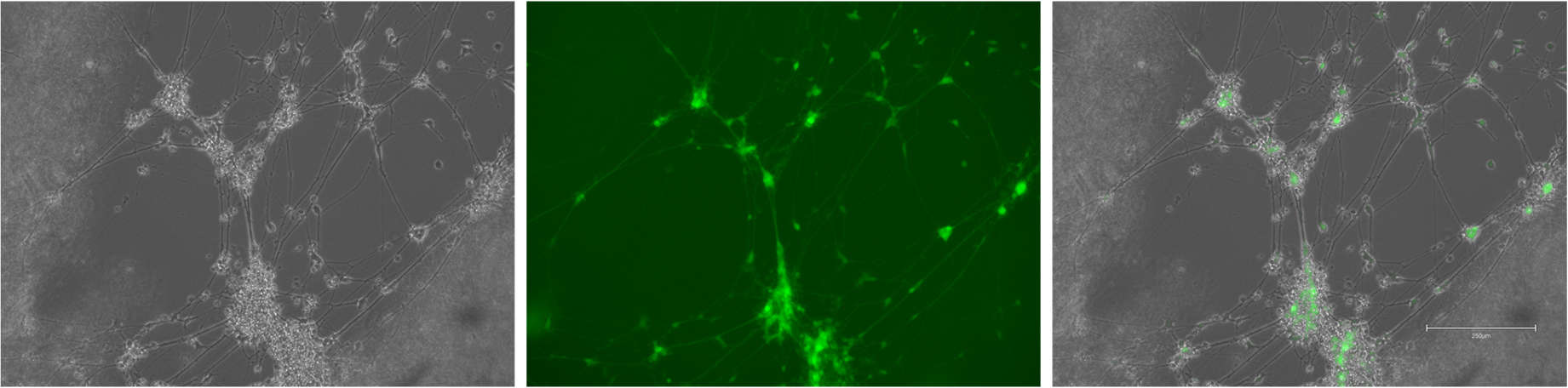
So when posed with this problem of developing a brain model, the first place I wanted to look to is the developing brain. Other 3D models of the brain often have issues with the maturity of the cells and networks, so taking hints from the timeline evolved over millions of years and the cues that facilitate it gave a good starting point. The cues can be divided into three main categories: molecular signalling, architecture and the extracellular matrix.
The extracellular matrix is often overlooked but it provides the basis of my modelling approach. Occupying the space between the neurons and glia in the brain, it is a dynamic and complex polymer network that plays many roles. To recapitulate this, I will be using a hydrogel base for my model – its easily modified to the characteristics I want and will allow me to print my construct with a bioprinter. This is the foundation for the rest of the project, so my work so far has been all about optimising, printing and troubleshooting the gel component.
The architecture of the brain can be considered at multiple levels from the whole brain down to the cellular network, but by focusing on modelling the cortex – the region responsible cognition – you can pick out some key features. In development, the process of building the cortex (corticogenesis) sees the movement of immature neuronal cells to form 6 distinct layers. With a combination of scaffold-like radial glia cells and molecular cues released from Cajal-Retzius cells, the immature neurons are guided into position. In my modelling, I am considering how to print a microscaffold of channels that will behave like radial glia, guided by a molecular gradient.
Molecular signalling also refers to the growth factor gradients that are crucial in neural development – factors like BMP-7, VEGF, BDNF, laminin, nectin, N-cadherin which all have varying roles – so optimising the combination, concentration and release of the chosen growth factors will be the next stage of my work.
In conclusion, the end goal of my project is to create a printable 3D construct, with a selected number of architectural and molecular cues that can support neurons and glial cells that can function as a network. The impact that work like this can have on the field is pushing the possibility of animal free testing of effective neurodegenerative disease therapies closer to realisation.