From Clinic to Bedside: The Importance of Point-of-Care Testing
By LifETIME CDT Student: Bianca Castelli (NUI Galway)
Suffering from a neurodegenerative condition is already strenuous. On top of dealing with the hardship of a disease, countless visits to several clinicians can make things even worse. For many people living with these conditions, getting out of the house and into a clinic is not an easy task. Some may not have an easy means of travel, or a caregiver helping them; others may live in secluded areas, far away from the closest specialist. For many, the simplest tasks can be very distressing. So, what if there was a way to bring clinical testing directly to the home or a doctor’s office?
Point-Of-Care (POC) testing, often referred to as bedside testing, is an umbrella term that generally refers to any type of diagnostic, monitoring, and screening technique that can be performed at the patient’s “point of care”, that is, the location of the patient at the time of care. Although the acronym POC might sound very unfamiliar to some, the technology is more common that it seems. From blood pressure monitors, to blood glucose measurement, pregnancy and ovulation tests, and many more, these are all examples of POC tests that have been used in primary care for years. So, what are the advantages of these devices? First of all, POC testing is a much faster way to get clinical results, as the site of testing is the same as that of sampling. And, speaking of samples, these tests are designed to be as non-invasive as possible, often only requiring accessible body fluids, or finger-prick blood. On top of all of this, POC testing can be performed by anyone, regardless of their skills or previous lab training.
Keeping all of this in mind, developing a POC device capable of monitoring neurodegeneration sounds like a very good idea. For people living with a neurological condition, one of the main struggles is accessing regular monitoring, which in turn helps clinicians decide on the right medications at the right time. As an example, for many people living with primary progressive multiple sclerosis (PPMS) the choice of disease modifying treatments is already limited to a couple of drugs. Additionally, a suitable administration protocol or dosage regiment can be a big challenge for doctors to solve.
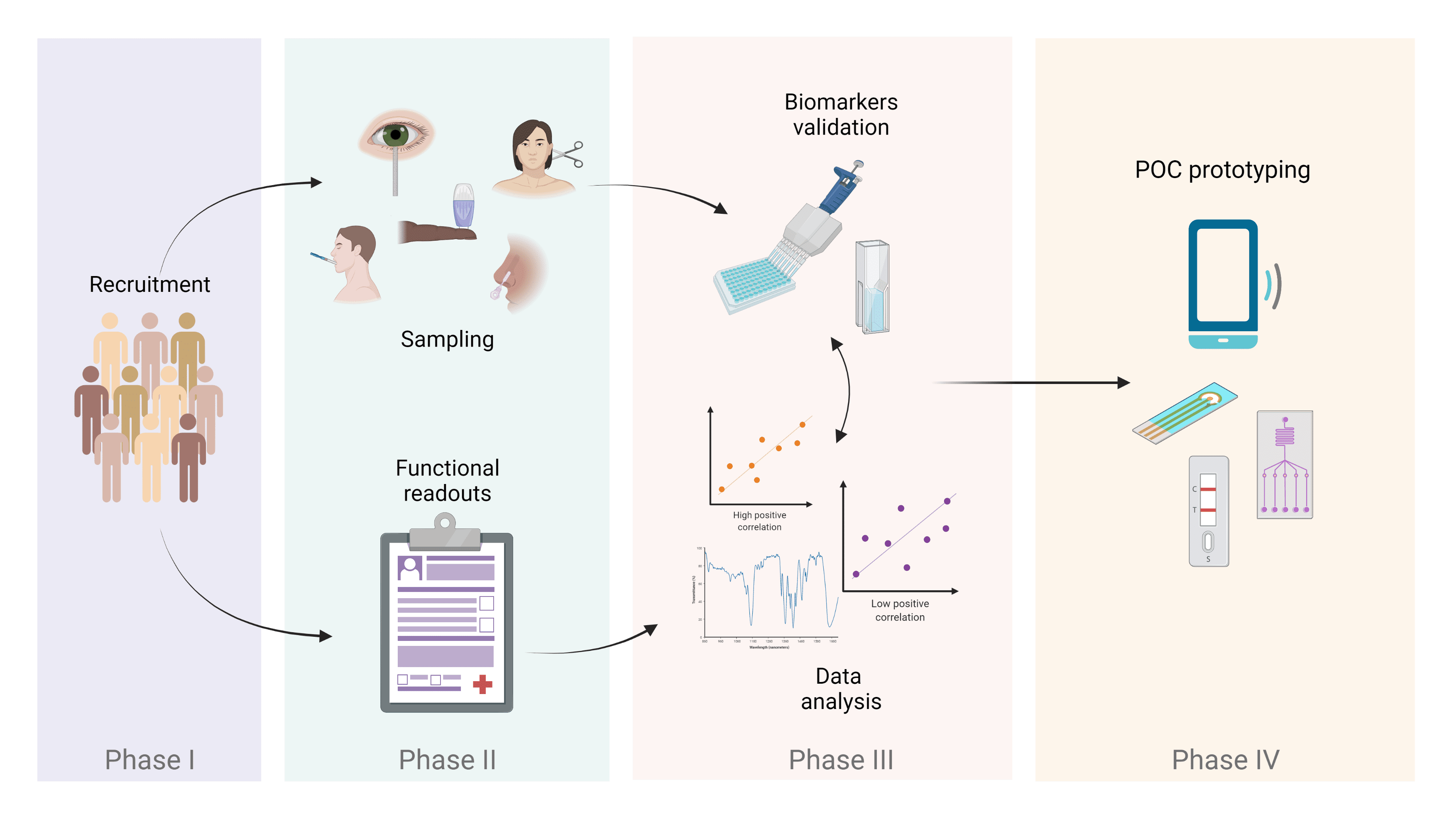
With this project, we plan to develop a POC device for clinicians and patients that can be used as a form of rapid disease monitoring and an alternative to more invasive and time-consuming techniques. In order to develop the technology, first we will need to define our biological markers of neurodegeneration. Think of these molecules as cues in an investigation for diagnosis or determining how disease is progressing. In very few cases, some cues are quite evident: their very presence can lead to only one conclusion. However, in the case of neurodegenerative diseases, we can’t rely on a single cue, but instead we must explore a set of candidate molecules. For this purpose, this study will involve a large cohort of people living with MS, where we aim to find our cues. To make this happen, we will take several body fluids samples (i.e. saliva, nasal secretion, tears, finger-prick blood) and try different assays to scout for molecules. Once we have established the right fluid (or fluids!) and quantified the biomarkers, we can integrate all into a novel POC device. We hope that this next-generation POC testing will benefit all MS (as well as other neurodegenerative diseases) community stakeholders, leading to an improved quality of life for all patients.
Schematics of the different phases involved in the project.