Lateral flow tests – they’re not just for COVID-19!
By LifETIME CDT Student: Rory Barnes (He/Him) (University of Glasgow)
Remember all those expired COVID-19 lateral flow tests you’ve got hidden in a cupboard somewhere? It might surprise you to find out that technology has been around a while, and we’re still working on improving it. Lateral flow tests first hit the market in 1980’s in the form of the at-home pregnancy test, Clearblue. Since then, the same technology has been applied to the detection of several infectious diseases, but really hit the headlines during the SARS-CoV-2 pandemic. The advantages are clear. They’re cheap, they’re fast, and they’re easy.
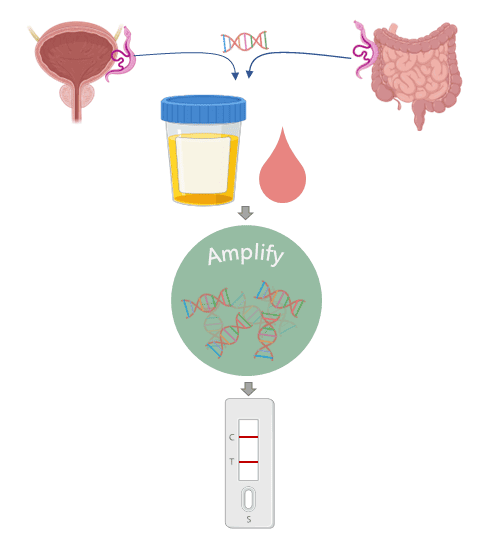
The tests detect certain molecules which are only found in the body fluids during an infection. These molecules might be produced by the parasite itself (antigens) or by the body in response to the infection (biomarkers). The problem is, for some diseases there might not be enough of these molecules for the test to pick up. This is where DNA comes in.
We’ve all got DNA. Me, you, the postman, the cat, and also the nasty parasites that infect us. Just like the antigens, the parasite will release its DNA into the bloodstream, where it can then get into all the other body fluids. However, there are still only tiny amounts of it, so how do we detect it? Nucleic acid amplification is a technology that can recognise a specific DNA sequence and copy it over and over again until you have millions of the same strand of DNA. Therefore, we have a way to turn the tiny amounts of DNA released from the parasite into an amount that can be detected by the lateral flow strip.
So now we can add another advantage to our list: Cheap, fast, easy, and also sensitive. Together, these factors make this technology well suited for diagnosing diseases that affect people in areas where access to expensive diagnostic labs isn’t available. For example, we are developing this technology for a disease called schistosomiasis which is caused by an infection of parasitic worms found in dirty water. It mainly affects children in low resource communities across Sub-Saharan Africa. ‘Traditional’ lateral flow tests aren’t sensitive enough to detect these worms, but currently available DNA tests are too expensive and complex to be used in these areas. We’re hoping that bringing the technologies together will provide a cost-effective tool for public health agencies in endemic countries to perform surveillance of schistosomiasis. If they can better understand where the burden of these infections is located, they can target the right children, with the right drugs, at the right time.