Joints in the lab
By LifETIME CDT Student: Maria Laura Vieri (University of Glasgow)
Hi everyone, I am a 1st year LifETIME CDT student. My PhD project is focused on the development of 3D hydrogel-based models of bone and cartilage, which can be used to evaluate the differentiation of induced pluripotent stem cells (iPSCs) into relevant cell lineages. Over the coming years, I also aim to integrate the CRISPR-Cas9 system, in an attempt to study osteoarthritis related pathways, in particular the role of proteinase activated receptor-2 (PAR-2).
Osteoarthritis is not uncommon, with more than 10% of people above 60 years old experiencing symptoms1. In fact, I would be surprised in many of you don’t have a family member who complains about feeling joint discomfort, and how this effects their everyday activities. Osteoarthritis is characterized by synovial membrane inflammation, degeneration of the articular cartilage and underlying bone, and although we don’t always know the exact reason for disease initiation and progression, it can occur due to “wear and tear” or joint injuries1,2. For this reason, it can also affect younger individuals, especially athletes3. Depending on the severity and extension of the pathology, treatment usually relies on relieving pain and stiffness1,4, with surgery being considered for the more severe cases. However, current surgical repair techniques are not able to stop the disease development or reduce its progression2 and therapy results are often unsatisfactory1.
Osteoarthritis has been shown to be an extremely complicated disease, in which not only the immune system is involved, but also the nervous system and the metabolism of the whole joint (cartilage, subchondral bone and synovium) seem to play a pivotal role2,4. A deeper knowledge of these mechanisms is thus essential for the development of future and more efficient therapies. Animal models are often used to investigate osteoarthritis pathways and treatments, before moving to human clinical trials5. Murine models are especially interesting for this kind of disease as their musculoskeletal system develops rapidly, allowing for a great number of analyses to be performed in a limited period of time. This reduces costs as well. However, apart from ethical concerns about animal testing, mice are not humans. Despite their closeness to our species, murine physiology and anatomy is different from ours, and this makes them an imperfect model. This means that, often, successful treatments in mice do not give the expected results in humans, especially in the long-term5. This is one of the reasons why research is moving towards finding new models that emulate human biology. Here is where tissue engineering joins the game.
Tissue engineering is defined as “an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ”6. These substitutes have already been used in cartilage surgery, to create surgical implants that allow not only the repair, but also the regeneration of damaged areas2. This research field also investigates the development of human tissues in vitro (that means they are created in a laboratory, out of a living organism), that mimic what naturally occurs in the human body7. Ideally, these models not only may be cheaper than animal breeding, but are also relatively easy to optimise in order to reduce the dissimilarities between the artificial model and the real pathology8. These models comprise three essential elements9:
- I will use iPSCs (reprogrammed stem cells that are pluripotent) that can essentially, given the right conditions, turn into any cell in the body; included cartilage cells (chondrocytes) and bone cells (osteocytes)10.
- Biomaterial, in my case hydrogels. Hydrogels are swollen polymer networks with characteristics close to those of the extracellular matrix that cells produce. These biomaterials can thus be tuned to create an environment in which stem cells can survive, proliferate, differentiate (mature into chondrocytes or osteocytes) and maintain their characteristics11.
- These include biochemical molecules that are necessary for a correct and stable stem cell proliferation and differentiation (i.e. specific growth factors), but also appropriate oxygen concentrations and mechanical stimuli12.
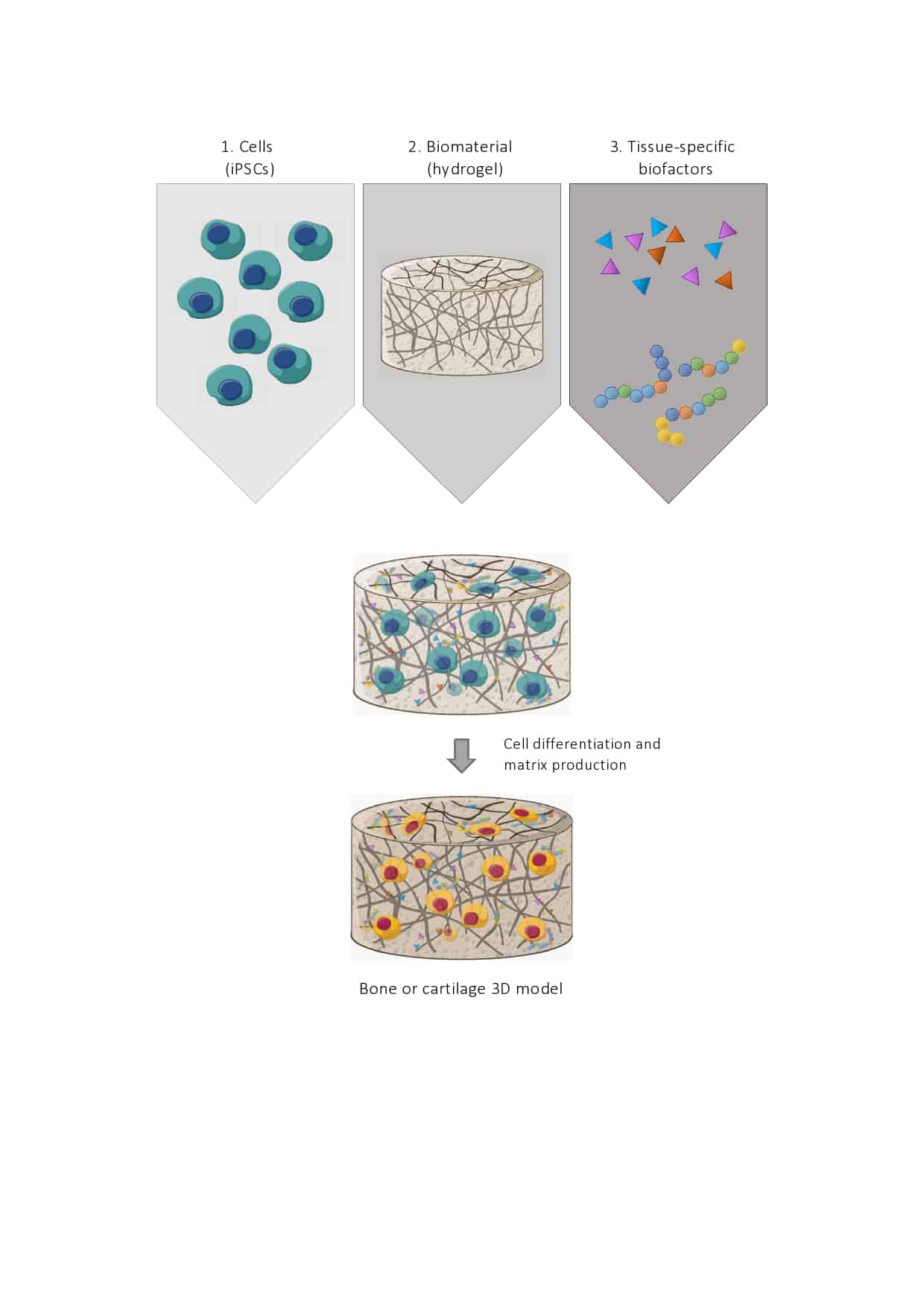
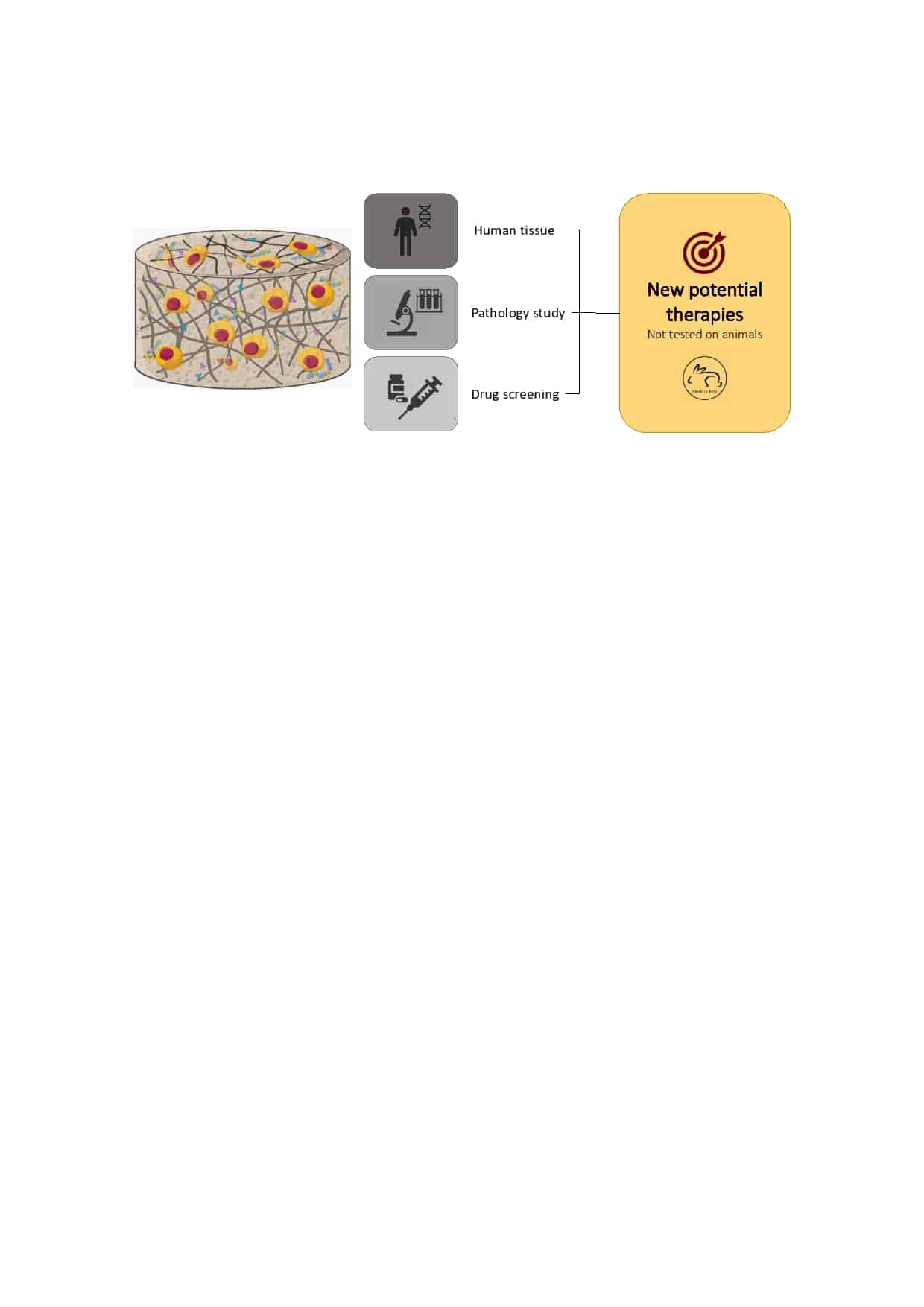
The initial aim of my work is to design the appropriate surrounding for iPSCs’ differentiation into chondrocytes and osteoblasts, using hydrogels. Once able to reproduce bone and cartilage-like tissues, I will use a known bacterial system for genetic engineering (CRISPR-Cas9) to study PAR-2, a receptor that has been shown to be essential for the generation of mineralised bone13.
My work will not only allow us to move away from animal use in scientific research, but it will also provide a more reliable model to study cartilage and bone related pathologies, potentially also fracture healing. Future researchers will be able to use these models to investigate and find new and more efficient therapies.
References
- Wu, Y., Goh, E. L., Wang, D. & Ma, S. Novel treatments for osteoarthritis: An update. Open Access Rheumatol. Res. Rev. 10, 135–140 (2018).
- Kwon, H. et al. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 15, 550–570 (2019).
- Amoako, A. O. & Pujalte, G. G. A. Osteoarthritis in young, active, and athletic individuals. Clin. Med. Insights. Arthritis Musculoskelet. Disord. 7, 27–32 (2014).
- Conaghan, P. G., Cook, A. D., Hamilton, J. A. & Tak, P. P. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 15, 355–363 (2019).
- Bapat, S., Hubbard, D., Munjal, A., Hunter, M. & Fulzele, S. Pros and cons of mouse models for studying osteoarthritis. Clin. Transl. Med. 7, 36 (2018).
- Langer, R. & Vacanti, J. P. Tissue engineering. Science (80-. ). 260, 920 LP – 926 (1993).
- Jensen, G., Morrill, C. & Huang, Y. 3D tissue engineering, an emerging technique for pharmaceutical research. Acta Pharm. Sin. B 8, 756–766 (2018).
- Yeung, P., Zhang, W., Wang, X. N., Yan, C. H. & Chan, B. P. A human osteoarthritis osteochondral organ culture model for cartilage tissue engineering. Biomaterials 162, 1–21 (2018).
- Vinatier, C. & Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 59, 139–144 (2016).
- Abdal Dayem, A. et al. Production of Mesenchymal Stem Cells Through Stem Cell Reprogramming. Int. J. Mol. Sci. 20, 1922 (2019).
- Geckil, H., Xu, F., Zhang, X., Moon, S. & Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine (Lond). 5, 469–484 (2010).
- Donnelly, H., Salmeron-Sanchez, M. & Dalby, M. J. Designing stem cell niches for differentiation and self-renewal. J. R. Soc. Interface 15, (2018).
- Huesa, C. et al. Proteinase-activated receptor 2 modulates OA-related pain, cartilage and bone pathology. Ann. Rheum. Dis. 75, 1989 LP – 1997 (2016).








