Protein self-functionalisation: evolving the hydrogel paradigm
By LifETIME CDT Student: Alexandre Trubert (University of Glasgow)
The field of tissue engineering has greatly excelled in recent years, scientists are now able to heal a variety of body tissues ranging from bone defects to whole internal organs; the practice of tissue engineering pivotally relies on materials compatible with the body, termed biomaterials, that act as scaffolds to guide tissue regeneration. Engineers have a variety of biomaterials in their toolbox for ranging applications, however, the most widely used and versatile biomaterial in the field is the hydrogel.
As the etymology suggests, hydrogels are highly hydrated gel-like construct comprising a fiber network and come in various types, from synthetically produced to naturally derived, engineers have developed and documented a plethora of hydrogel systems, the latest trend being the self-assembling peptide hydrogel. A peptide hydrogel uses short amino acid sequences, termed peptides, as building blocks to construct the fiber network; these peptides are designed to spontaneously assemble upon introduction to water, eliminating the necessity of lengthy chemical reactions a classic hydrogel tacitly entails.
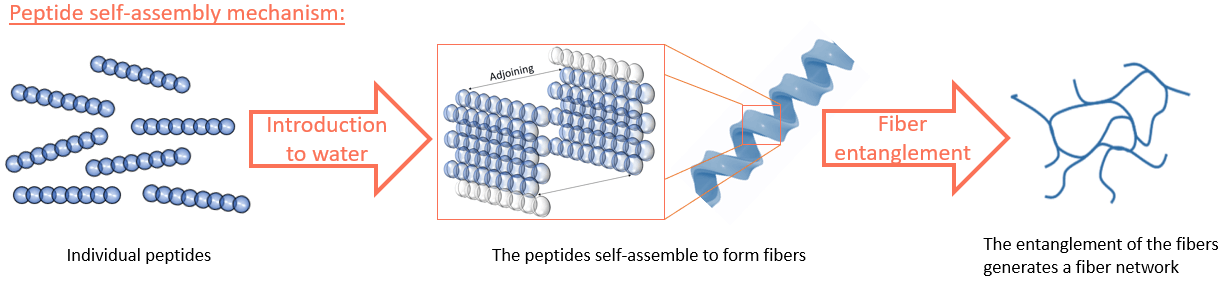
Now, an engineer’s perpetual ambition with hydrogels is their ‘functionalisation’, meaning the incorporation of natural proteins that will help the hydrogel boost cellular activity and ultimately, enhance its regenerative performance. On the other hand, that functionalisation procedure is intrinsically challenging to achieve; hence, scientists are searching for easier functionalisation methods that can be consistently reproduced.
In my project, I seek to exploit the self-assembly system of peptide hydrogels to allow what I call, the ‘self-functionalisation’ of these natural proteins, and offer an easy and reproducible alternative to hydrogel functionalisation, consistent only for the peptide hydrogel system. Allow me to elaborate, the advances of recombinant protein expression using bacteria allows us to produce a vast array of proteins in large quantities; accordingly, the sequence of the expressed protein can be meticulously tailored down to the single amino acid. Thus, the insertion of that amino acid sequence to the terminal of the protein of interest will proffer that protein with a ‘peptide tag’ that could act as a peptide building block. As such, by the embedment of the peptide tag of the protein in the peptide self-assembly that forms the fiber, I anticipate to innately tether the protein to the fiber network as the hydrogel is being formed, eliminating the need for post-production modifications.
Our focus at the Salmeron-Sanchez lab is to tether the fiber network of hydrogels with growth factors, potent molecules that can significantly enhance the developmental capacity of cells for regenerative purposes. Thus, the pilot protein we want to functionalise as a proof-of-concept to this project is fibronectin, a prominent natural protein comprising a series of regions; namely, the 12th to 14th regions of fibronectin, termed FNIII12-14, have been identified as promiscuous growth factor binding sites that can acts as anchor points for growth factor tethering.
Thus, through our collaboration with Manchester Biogel Ltd., a company that commercialises their own peptide hydrogel system, we are actively expressing the FNIII12-14 with the corresponding peptide tag at its terminal to manufacture the functionalised hydrogel according to the described approach.
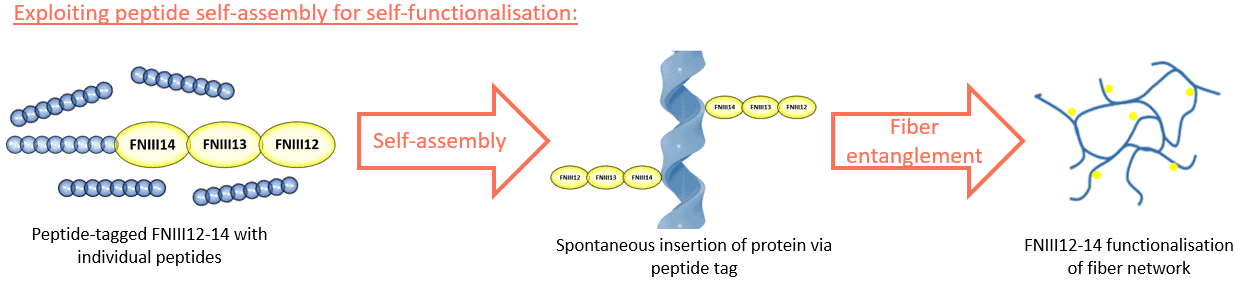
The proof-of-concept of this project paves the way for a wide array of applications; within the scope of FNIII12-14, engineers could use the functionalised construct to tether a library of growth factors that could influence and promote the activity of multiple cell types according to the employed growth factor combination, thus versatile for multiple tissue types. Outside the scope of FNIII12-14, the described strategy can be implemented for the functionalisation of other proteins that fill different functions, for instance cell binding sites or chemotaxis inducers to name just a few. All in all, the described construct and functionalisation strategy offers a reliable, reproducible, and versatile platform that can be added to the engineer’s toolbox to further advance the field of tissue engineering.








