Viscoelastic 3D Hydrogels: How and why are they better
By LifETIME CDT Student: Theodora Rogkoti (University of Glasgow)
The bone marrow is a specialised environment containing a complex set of cellular, biochemical, and physical signals that all work together to form the so-called “bone marrow niche”. These niche-specific cues regulate the localisation, function, and movement of Hematopoietic stem cells (HSCs), the cells responsible for developing all the mature cell types of the blood.
Until today, much of our understanding concerning the bone marrow microenvironment comes from animal models and cells grown on 2D surfaces. The former has not always been predictive of the processes taking place in the human bone marrow, while attaining an understanding of how the bone marrow works has always been challenging due to difficulties associated with visualising this environment in humans. On the other hand, although 2D in vitro studies have provided valuable knowledge, cells grown on 2D conditions experience different signals (e.g. surface topography, stiffness, cell-cell / matrix interactions) compared to cells within the body which are located within a 3D dynamic environment. Thus, there is an unmet need to create physiologically relevant, 3D in vitro models that will allow us to understand the bone marrow niche in health and disease and enable development of new therapeutics.
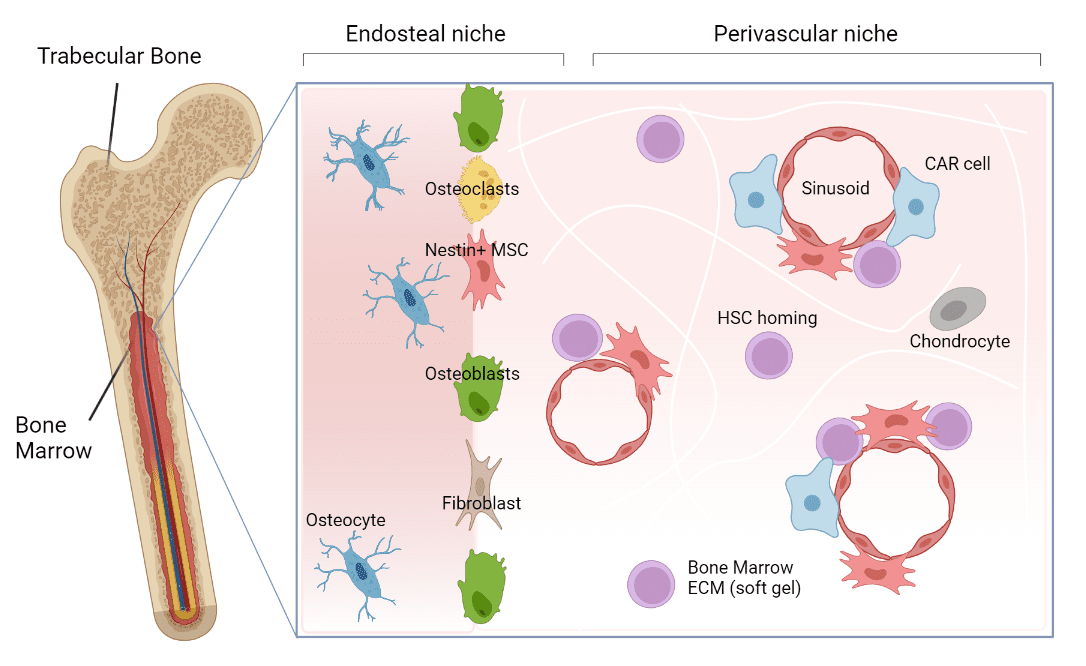
The bone marrow homes a variety of cell populations including, among many, endothelial cells, osteoblasts, fibroblasts, and Mesenchymal stem cells (Figure 1). These Mesenchymal stem cells are thought to be the most important associated cells for HSC function and are found at different areas within the niche. They secrete various nutrient-like factors important for HSC support and maintenance, creating an environment that facilitates a delicate balance between HSC self-renewal and maturation into blood-related cells.
Figure 1: The bone marrow niche. The bone marrow niche is a dynamic environment that supports the maintenance and movement of HSCs, the cells responsible for making all blood cells. Within the niche there are various areas that home different types of cells with the most important ones being Mesenchymal stem cells. These Mesenchymal stem cells secrete factors that help HSCs grow and mature.
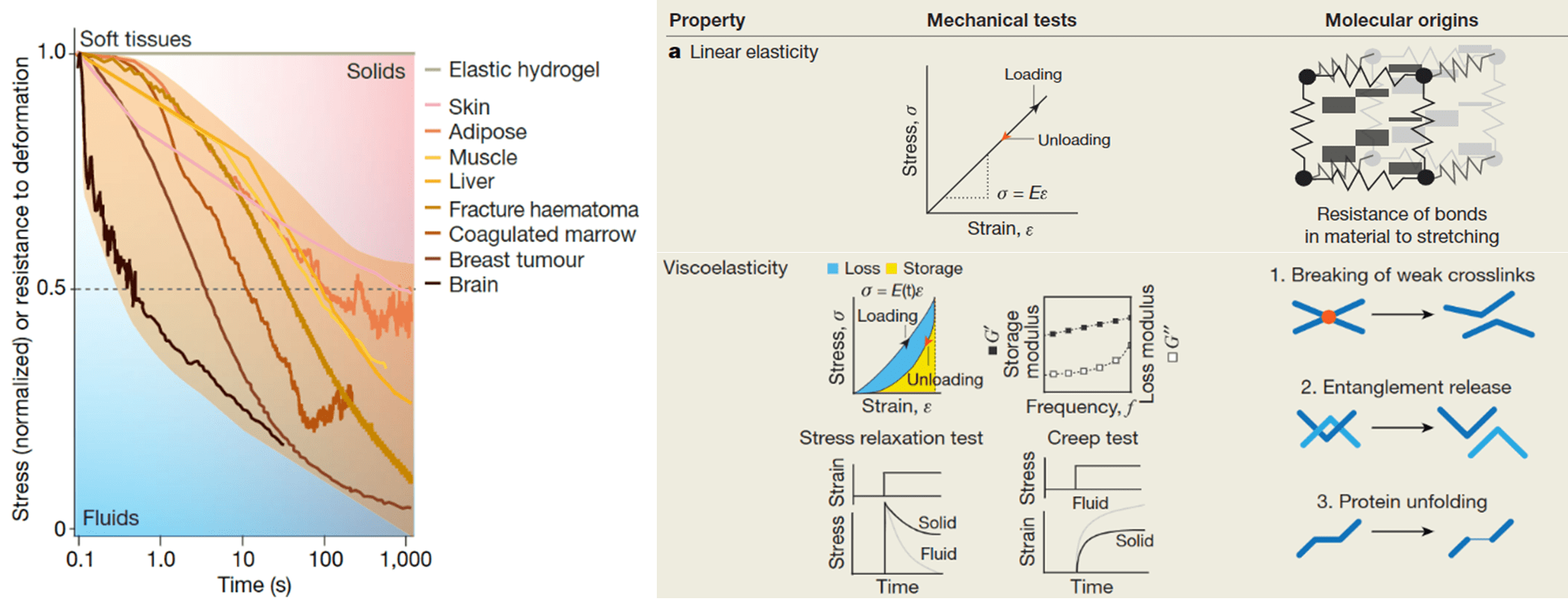
Furthermore, the matrix component within the bone marrow niche is also vital for appropriate cell function and maintenance. The extracellular matrix (ECM) is composed of many proteins that allow cells and nutrient-like factors to adhere and be presented to cells, respectively. These proteins form a space-filling nanoporous gel-like matrix, with a stiffness ranging from 0.2-25kPa, a factor important for homeostasis. However, while many researchers have tried to recreate the bone marrow niche using 3D elastic hydrogels that match the stiffness of the in vivo environment, the bone marrow ECM is not purely elastic. It exhibits complex mechanical behaviour, including a time-dependent response to deformation, a phenomenon known as viscoelasticity (Figure 2). In other words, when stretched, ECMs within our tissues do not respond like a string, whereby removal of the applied stress allows the material to return to its initial position almost instantly. They instead resemble the mechanical properties of silly putty, which returns to its initial position slowly. Interestingly, an emerging body of evidence has recently demonstrated that the viscoelastic behaviour of ECMs affects cell behaviour, sometimes in unexpected ways which do not match the findings of previous research which was based on purely elastic substrates. Considering this, the development of biomaterials with tuneable viscoelasticity may be transformative in improving our understanding of the processes underlying the physiological and pathological bone marrow niche.
Figure 2: Mechanical behaviour of different biological tissues. Biological tissues and ECMs in the body are not linearly elastic solids. They exhibit complex behaviour including stress relaxation in response to a constant strain, a sign of their viscoelastic nature. This means that apart from storing some energy, in the same way that solids do, they also dissipate energy, frequently in the form of heat, like fluids.
In this project, we aim to develop a novel physiologically relevant bone marrow model using synthetic viscoelastic hydrogels to investigate the healthy and diseased bone marrow niche. To do this, viscoelastic hydrogels will be initially developed and their mechanical properties, including the stiffness and time-dependent viscoelastic properties, will be assessed. Following functionalisation with ECM proteins, the bioactivity of viscoelastic hydrogels will be assessed using Mesenchymal stem cells in the form of spheroids considering their important role in HSC maintenance within the niche. The system will be used to interrogate HSCs within the model, their ability to stick to the hydrogels as well as their motility and migration outside the bone marrow niche – as a sign of cancer. It is also anticipated that the model will serve as a platform to study leukaemia processes in 3D human in vitro conditions as well as the effect of ECM time-dependent properties on malignancy, reducing the need for animal models. Finally, nutrient-like factors will be introduced into the system using Polyhedrin Delivery Systems, protein micro-crystals that allow the encapsulation and release of these factors over a sustained period of time. Ultimately the development of such a model will aim to drive the replacement and reduction of animals used and further our knowledge and treatment of bone marrow-related diseases.