How can we use magnets to grow bones?
By LifETIME CDT Student: Emma Kelly (University of Glasgow)
Did you know bone tissue is the second most transplanted tissue worldwide? We use bone transplants, called bone grafts, to treat various types of damage to bones such as fractures that won’t heal, to replace part of a bone that was surgically removed and skeletal abnormalities. However, bone grafts depend on using the patient’s own healthy bone, or donor bone, to replace the damaged bone; this comes with many problems.
Bone tissue engineering is working to replace and enhance current methods of bone regeneration and repair, by using bone tissue grown in a laboratory environment, rather than using a patient or donor’s healthy bone. Advancements in bone tissue engineering have been supported with the development of biomaterials. Biomaterials are natural or man-made materials that interact with a biological component, e.g., contact lenses are composed of biomaterials and designed to be worn on the surface of the eye. A common type of biomaterial is a hydrogel, a material that maintains a 3D structure with a high-water content. My project aims to develop a hydrogel that will support and accelerate the growth of new bone tissue using magnetic stimulation.
Magnetic fields are one of the many forces that are exerted on developing tissues. Even before we are born, there are many forces and stimuli that influence our growth and development such as biochemical and mechanical forces. As we learn more about these forces, we can use similar approaches in the lab to support the development of lab grown tissue. My project aims to utilise magnetic stimulation to help develop bone tissue engineering models.
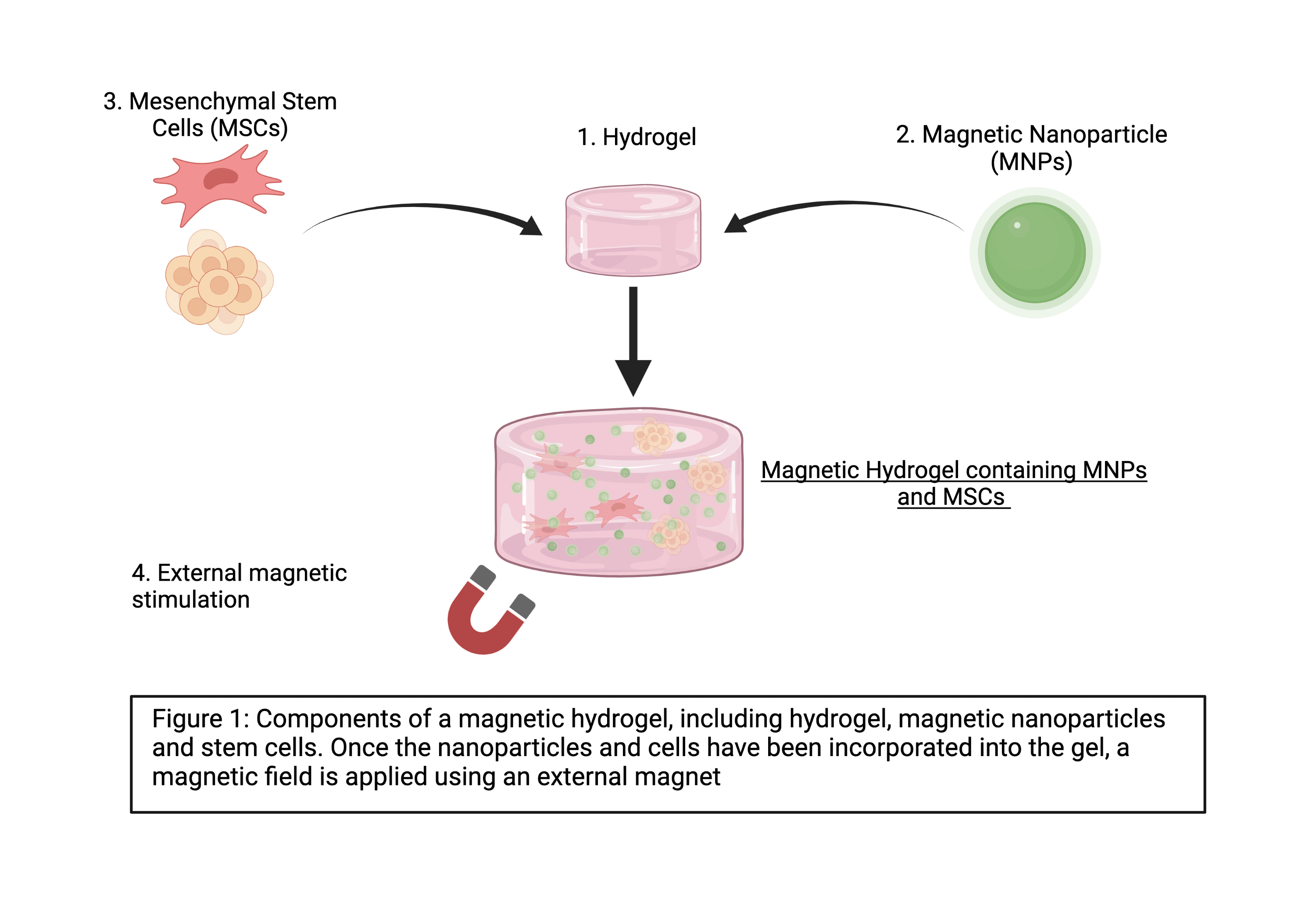
I’ll be developing a magnetic hydrogel to support bone tissue engineering. The design will focus on four key components:
- Hydrogels
- Magnetic Nanoparticles
- Stem Cells
- An External Magnetic Field
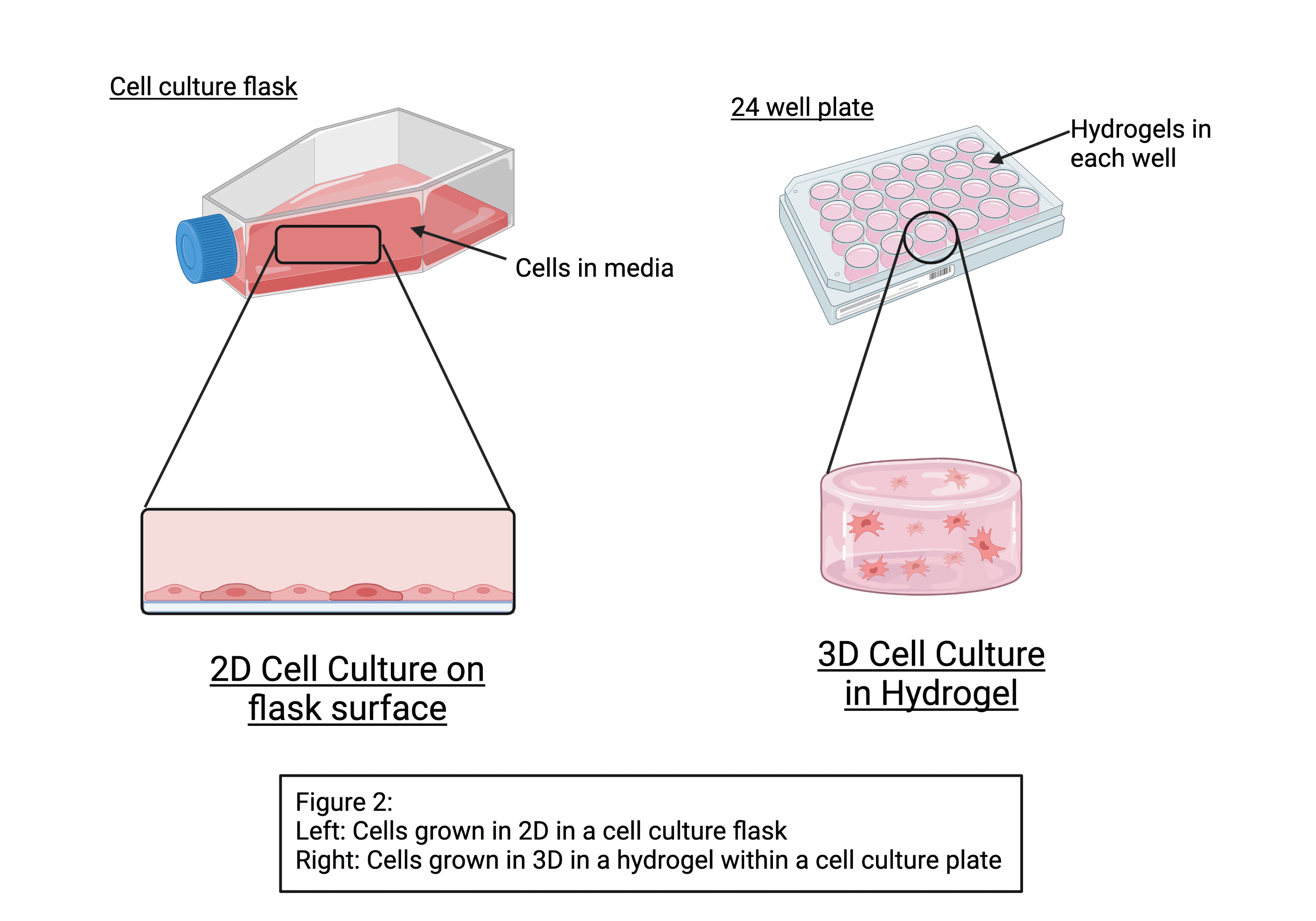
In our body, our cells grow in 3D within an extracellular matrix. When we grow cells in the lab, we tend to grow them in flasks and dishes in 2D. Hydrogels are biomaterials, which we can use to grow cells in 3D. Initially, I’m working with premade peptide hydrogels supplied by a company called Manchester Biogel.
Nanoparticles are very small particles that range between 1-200nm in size. I’m using magnetic nanoparticles composed of an iron oxide. I’ll be incorporating the nanoparticles into the hydrogel and using fluorescent nanoparticles initially to see how well they disperse in the gel.
I’m using mesenchymal stem cells which have the ability to develop into bone tissue, through a process called osteogenesis. I’ll be adding them into the hydrogel as single cells, but also as spheroids – which are 3D groups of cells grown together.
An external magnetic field, similar to a very strong fridge magnet, will be used to stimulate the magnetic nanoparticles within the hydrogel. Without the external magnetic field, the nanoparticles don’t have magnetic stimulation, but when an external magnetic field is applied, they become magnetically active. This allows us to control how much magnetic stimulation the cells receive.
By developing a magnetic hydrogel, we hope to accelerate and improve bone tissue development. Bone tissue engineering is working to support traditional treatment methods to improve patient outcomes. If our magnetic hydrogel does promote and accelerate osteogenesis, we can use this biomaterial in bone tissue engineering research both in the lab and potential in clinical settings.