3D breast cancer-on- a-chip model
By LifETIME CDT Student: Yashna Chabria (NUI Galway)
 While patients diagnosed with early stage breast cancer have a good outcome, it is more challenging to treat once the tumor has spread and so novel therapies are urgently required. Usually, the first step in breast cancer spread is to the adjacent lymph nodes. An increase in the number of diseased lymph nodes results in increased relapse rates that in turn results in a decline in patient survival. Understanding the factors controlling this initial spread is hence critical for the development of new therapies.
While patients diagnosed with early stage breast cancer have a good outcome, it is more challenging to treat once the tumor has spread and so novel therapies are urgently required. Usually, the first step in breast cancer spread is to the adjacent lymph nodes. An increase in the number of diseased lymph nodes results in increased relapse rates that in turn results in a decline in patient survival. Understanding the factors controlling this initial spread is hence critical for the development of new therapies.
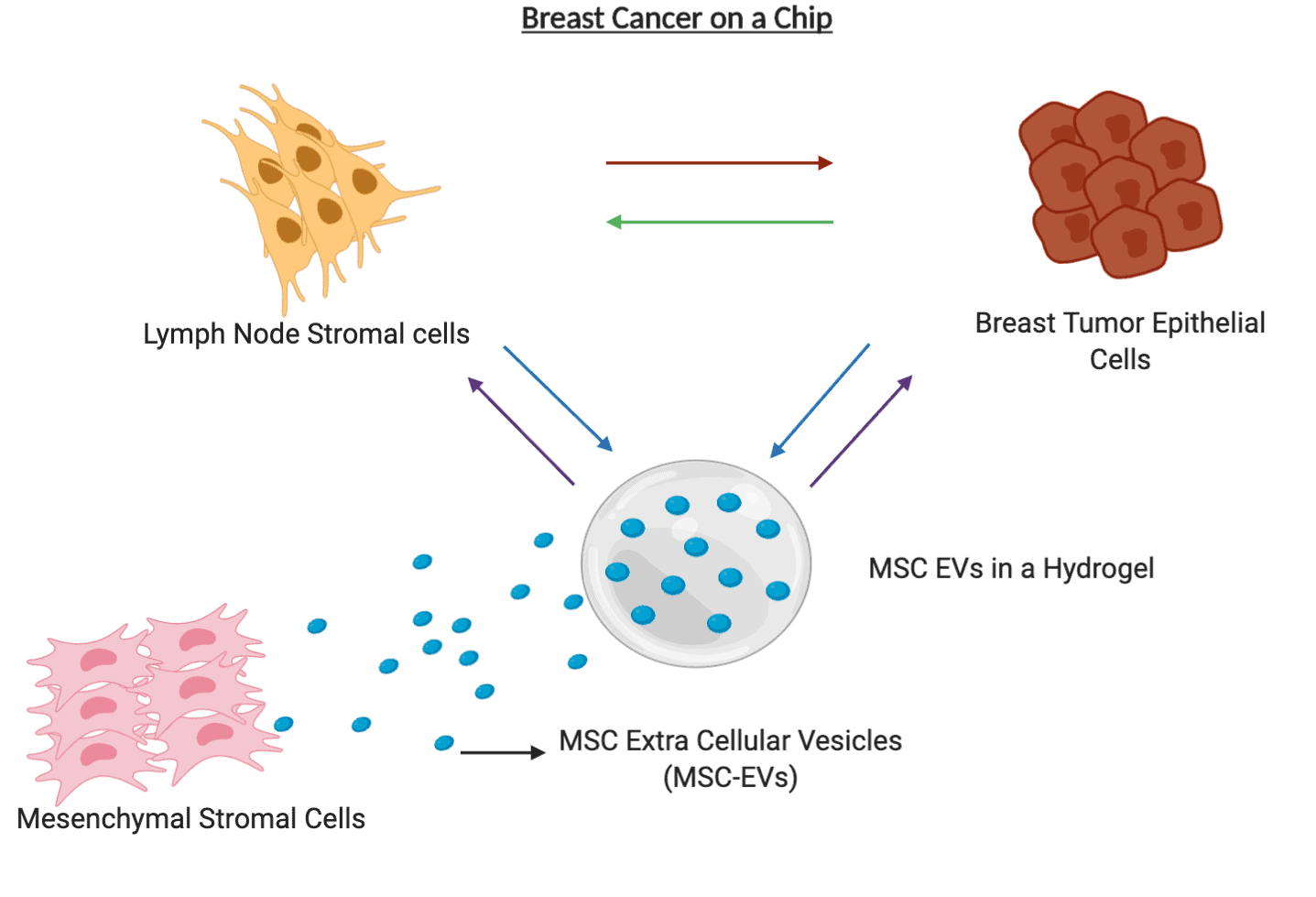
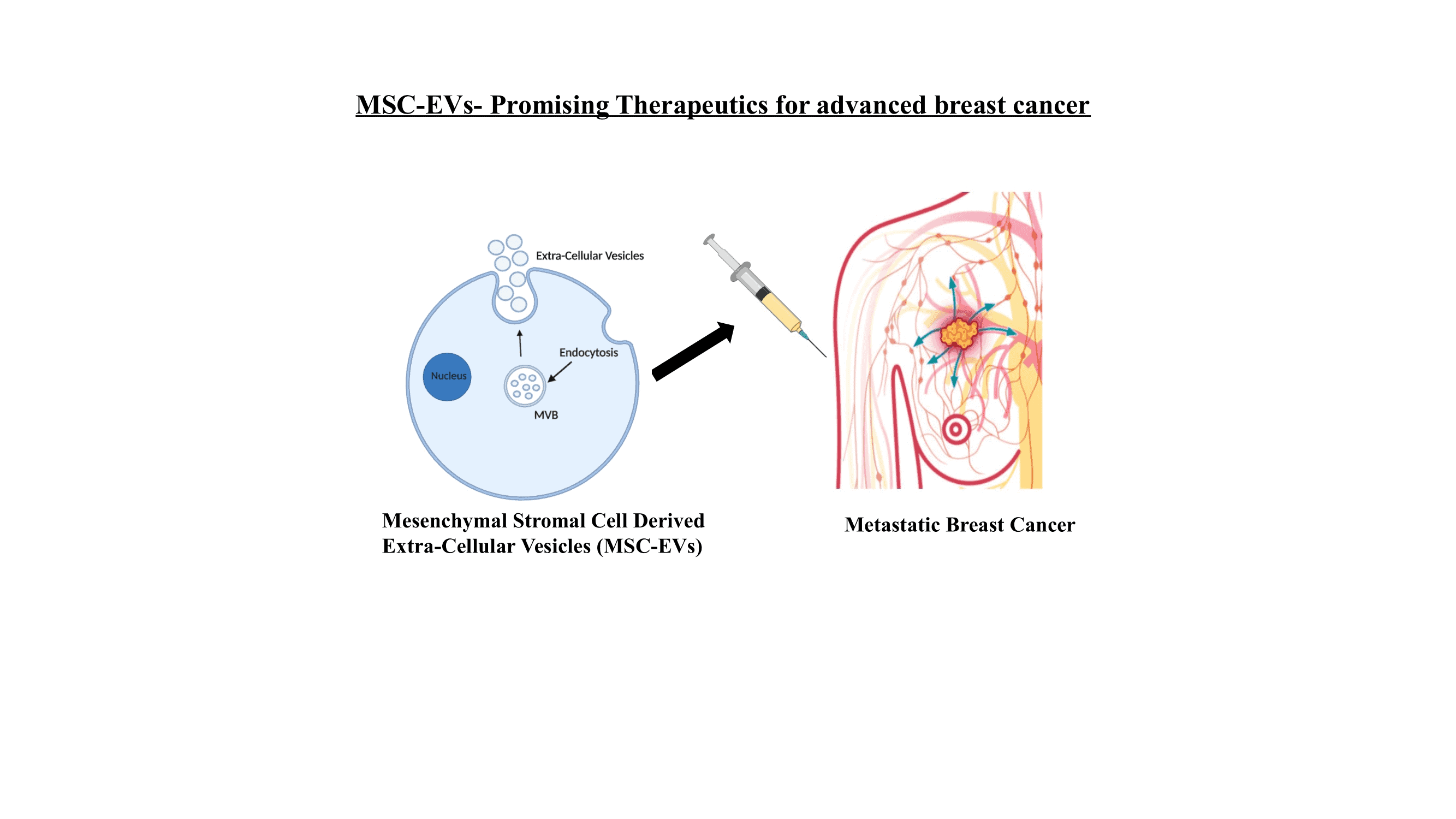
Mesenchymal Stromal Cell (MSC) therapy has been in the limelight for quite some time now for the treatment of several diseases, due to their regenerative capacity of these cells. MSC wound healing properties are elicited by the release of several growth factors. However, these MSCs are also well known for their ability to travel specifically to the sites of tumours, since they resemble wounds. MSCs can also bypass the immune system, which is important to avoid a toxic response in patients. However, in the cancer setting growth factors released by MSCs would potentially support tumour growth. MSCs, like all other cells, are known to secrete tiny extracellular vesicles (EVs) that are nanometer- sized tiny bubbles. Previously thought to be tiny disposal sacs of minimal significance, these are now known to be the fingerprint of the parent cell, meaning they bear the characteristics of the cells they are released from. Therefore, MSC-derived EVs (MSC-EVs) may be able to find tumours and lymph node metastases by bypassing the host system just like the parent cell and would avoid any potential for pro-tumorigenic effects of the MSCs. These lipid vesicles have huge potential as they can transport therapeutic drugs or genetic material specifically to the tumor cells, while avoiding healthy tissues. Therefore, the sustained release of these vesicles would be a potential therapeutic option for metastatic breast cancer patients.
A preliminary study carried out by my research group, indicated how the MSC-EVs carrying tumor ameliorating micro-RNAs resulted in reduced tumor growth. However, we have yet to understand the interactions of these EVs with the tumor microenvironment and the factors involved in their homing capacity to the tumor site. Therefore, my project aims to develop a clinically relevant cancer-on-a-chip model to study trafficking of these therapeutic MSC-EVs towards the breast tumours and lymph nodes metastases. The MSC-EVs will be packed in a hydrogel in order to facilitate their sustained release, potentially allowing for sustained therapy in patients with advanced disease. The cancer-on-a-chip set up will help us analyse these interactions in real time and also minimize the use of animal models. The microfluidic device will also help us to visualize the interactions between the tumor and the lymph node cells that predominantly occurs in breast cancer patients when the disease starts to spread. This will support improved understanding of breast cancer metastasis and allow us to harness this knowledge to develop a novel treatment for patients with advanced breast cancer.
Reference for the images: https://biorender.com/